When Will Gene Therapy Be Available As A Treatment
Gene therapy is in clinical development and is therefore not yet widely available as a treatment option. Ongoing clinical trials involve a small number of patients and have strict adherence to specific protocols. These are run from only a few clinical centres. Further research and wider clinical trials are, of course, required to obtain approval from regulators before this type of treatment can be more widely used.
Key aspects researchers are addressing currently include:
- the possibility of regulating the amount of therapeutic material that is present at any time so that the best results can be achieved
- the possibility of targeting brain cells through less invasive delivery routes, e.g., by injections into the systemic circulation or the cerebrospinal fluid
- ensuring that the viruses introduced are safe and do not induce an immune reaction
- ensuring that the treatment itself does not induce unexpected detrimental effects due to the ability of inserted therapeutic genes to alter things that were not intended to be changed.
Progress In The Treatment Of Parkinsons Disease
Despite the fact that 200 years passed since the discovery of PD, it was not until later in the 20th century that progress in the treatment of PD was achieved, predominantly due to the limited understanding of PD pathophysiology. Given Carlssons discoveries of DAs involvement in the 1950s, it became clear that PD development involved dopaminergic cell death and a decrease of DA in the striatum and other structures of the forebrain. The first steps towards treatment were made by Carlsson , who proposed targeting this DA deficiency to facilitate symptom reduction.
Prevail Therapeutics Provides Clinical Advancement Update On Pr001 For The Treatment Of Parkinsons Disease With Gba1 Mutations
Company Will Present at Cowen Healthcare Conference Today
NEW YORK, March 03, 2020 Prevail Therapeutics Inc. , a biotechnology company developing potentially disease-modifying AAV-based gene therapies for patients with neurodegenerative diseases, provided an update today on the clinical advancement of its gene therapy program PR001 for patients with Parkinsons disease with GBA1 mutations . Enrollment in the PR001 Phase 1/2 PROPEL clinical trial is progressing, patient dosing continues, and the Company is on track to report interim data on a subset of patients in the second half of 2020.
The Company will present on its clinical progress at the Cowen & Co. Annual Healthcare Conference in Boston today.
We believe the PROPEL trial makes PR001 the first potentially disease-modifying gene therapy for PD-GBA patients to enter clinical trials. Its ongoing progress brings us a step closer to new treatment options for patients living with PD-GBA, said Asa Abeliovich, M.D., Ph.D., Founder and Chief Executive Officer of Prevail. We are excited about the potential of PR001 to slow or stop disease progression for PD-GBA patients.
In addition to the PROPEL clinical trial for patients with PD-GBA, PR001 is also being developed for neuronopathic Gaucher disease, a devastating disorder that shares the same underlying genetic mechanism. In December 2019, the Company announced that its IND for PR001 for the treatment of neuronopathic Gaucher disease is active.
Also Check: Parkinson’s Donations In Memory Of
Treatment Of Parkinsons Symptoms With The Dopamine Precursor L
Based on Carlssons discoveries, Hornykiewicz and colleagues developed the treatment of PD with the DA precursor, L-DOPA . This approach compensates for decreased DA by promoting DA synthesis in midbrain DA neurons. As evidenced in several pop-culture pieces, such as the award-winning motion picture Awakenings starring Robin Williams and Robert De Niro and based on the novel of the same name written by Oliver Sacks, the success of this approach in patients with PD was dramatic and often quite rapid . Despite these dramatic effects, it was reported that L-DOPAs effects were often inconsistent, even within the same patients, and often eventually induced profound and intolerable side effects such as dyskinesia, motor fluctuations, and various emotional disturbances and psychiatric problems . Furthermore, all the clinical benefits of the treatment are eventually reverted with a continuation of dopaminergic neuronal death, as L-DOPA administration does not halt disease progression . However, despite these limitations, the improvement seen in some patients is so pronounced that these downsides do not prevent its use. Indeed, after almost 60 years, L-DOPA remains the gold-standard medication for PD .
An Update On Gene Therapy Approaches For Parkinsons Disease: Restoration Of Dopaminergic Function
Issue title: Repairing the Parkinsonian Brain
Guest editors: Anders Björklund, Bastiaan R. Bloem, Patrik Brundin and Howard Federoff
Article type: Review Article
Authors: Van Laar, Amber D.a b | Van Laar, Victor S.c | San Sebastian, Waldya e | Merola, Aristided | Elder, J. Bradleyc | Lonser, Russell R.c | Bankiewicz, Krystof S.c e *
Affiliations: Asklepios BioPharmaceutical, Inc., Columbus, OH, USA | Department of Neurology, University of Pittsburgh Medical Center, Pittsburgh, PA, USA | Department of Neurological Surgery, College of Medicine, The Ohio State University, Columbus, OH, USA | Department of Neurology, College of Medicine, The Ohio State University, Columbus, OH, USA | Department of Neurological Surgery, University of California San Francisco, San Francisco, CA, USA
Correspondence: Correspondence to: Krystof S. Bankiewicz, The Ohio State University, Department of Neurological Surgery, N1018 Doan Hall, 410 W. 10th Ave., Columbus, OH 43210, USA. E-mail: .
Keywords: Gene therapy, Parkinsons disease, aromatic-L-amino-acid decarboxylase, glial cell line-derived neurotrophic factor, image-guided convection-enhanced delivery, clinical trial design
DOI: 10.3233/JPD-212724
Journal: Journal of Parkinson’s Disease, vol. 11, no. s2, pp. S173-S182, 2021
Abstract
Read Also: Vascular Parkinsonism And Cognitive Impairment
Gene Therapy Studies Underway In Parkinson Disease
Bayer provided an update on their phase 1 and 1b studies of dopaminergic neurons and GDNF gene therapy, respectively, for the treatment of PD.
This content originally appeared on our sister site, GeneTherapyLive. Read it here.
The first patient has been dosed in BlueRock Therapeutics’ phase 1 study of DA01 for the treatment of Parkinson disease , Bayer, the holding company of BlueRock, recently announced. Asklepios BioPharmaceutical , another subsidiary of Bayer, is also enrolling patients in its phase 1b clinical study of glial cell line-derived neurotrophic factor gene therapy for PD.1
The potential of BlueRock and AskBios clinical candidates to treat PD could be immense, said Wolfram Carius, head, cell and gene therapy, Bayer, in a statement.1 For the first time, it might be possible to stop and reverse this degenerative disease and truly help patients with their high unmet medical need. The start of clinical trials represents the beginning towards a truly breakthrough treatment option to dramatically improve the lives of patients.
The Future Of Research In Neurodegenerative Disease
Additional studies across the country are now using this new technique for other neurodegenerative diseases and neurologic disorders. The strategy allows researchers to evaluate the true extent of therapeutic delivery, eliminating the possibility that an effective therapy won’t work simply because it missed its mark.
“Mass General has a history of making safe but innovative advances in functional neurosurgery, and we’re well-positioned to be an international center of excellence for gene therapy in the brain,” Dr. Richardson says. “We have a lot of collaborators in other areas of neurology, psychiatry and the basic sciences with whom we can partner in the future to expand the application of this technique.”
Inquiries into this phase II clinical trial at Mass General can be sent to: , or call 617-726-2937.
Refer a patient to the Department of Neurosurgery
Contributors
Also Check: Parkinson’s Multiple System Atrophy
Frontiers In Aging Neuroscience
National Institute on Aging, National Institutes of Health , United States
Reviewed by
Northwestern University, United States
The editor and reviewers’ affiliations are the latest provided on their Loop research profiles and may not reflect their situation at the time of review.
Effects Of Gene Therapy May Be Long Lasting
We have evidence from a previous study that the gene therapy results in stable expression of the AADC enzyme, said Christine, referencing data demonstrating stability over a five-year duration. We believe that this treatment will allow these patients to more efficiently convert levodopa into dopamine, thereby obtaining greater improvements in mobility with each dose. Since many patients were able to substantially reduce the amount of Parkinsons medications, this gene therapy treatment may also help patients by reducing dose-dependent side effects, such as sleepiness and nausea.
The treatment was generally well tolerated, but one patient experienced a blood clot and irregular heart rhythm likely related to the surgery, said senior author Paul Larson, MD, of the UCSF Department of Neurosurgery.
A Phase II of the study of this gene therapy was recently launched and that study will allow us to better understand the safety and effectiveness of this treatment, Larson said.
While results of this phase I trial are promising, there are a number of non-motor features of Parkinsons disease that may develop over time, such as depression, as well as cognitive changes, said Christine. These conditions do not respond to levodopa and we do not believe that gene therapy will address them.
Funding: Michael J. Fox Foundation for Parkinsons Research and Voyager Therapeutics, Inc.
Don’t Miss: Parkinson’s Disease And Vision Problems
Strategies For The Treatment Of Parkinsons Disease: Beyond Dopamine
- 1Laboratorio de Neurobiología, Facultad de Ciencias de la Salud, Universidad San Sebastián, Concepción, Chile
- 2Department of Biological Sciences, University of Limerick, Limerick, Ireland
- 3Health Research Institute, University of Limerick, Limerick, Ireland
- 4Department of Psychology and Neuroscience, Center for Neuroscience, University of Colorado, Boulder, CO, United States
- 5Research & Development Service, Bay Pines VA Healthcare System, Bay Pines, FL, United States
Parkinsons disease is the second-leading cause of dementia and is characterized by a progressive loss of dopaminergic neurons in the substantia nigra alongside the presence of intraneuronal -synuclein-positive inclusions. Therapies to date have been directed to the restoration of the dopaminergic system, and the prevention of dopaminergic neuronal cell death in the midbrain. This review discusses the physiological mechanisms involved in PD as well as new and prospective therapies for the disease. The current data suggest that prevention or early treatment of PD may be the most effective therapeutic strategy. New advances in the understanding of the underlying mechanisms of PD predict the development of more personalized and integral therapies in the years to come. Thus, the development of more reliable biomarkers at asymptomatic stages of the disease, and the use of genetic profiling of patients will surely permit a more effective treatment of PD.
Direct Enzymatic Enhancement Of Dopamine Production
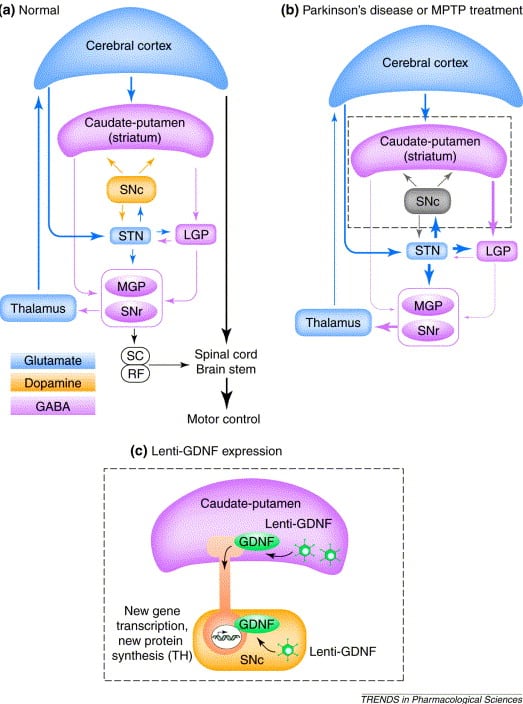
The most direct way of addressing DA restoration is to increase DA production at the site of greatest deficiencythe putamen. To this end, several gene therapy studies have focused on increasing expression of enzymes in the DA synthesis pathway, namely tyrosine hydroxylase , aromatic L-amino acid decarboxylase , and GTP cyclohydroxylase 1 .
Fig. 1
Preclinical studies in non-human primate parkinsonian models explored striatal viral transduction and expression of AADC alone, AADC with TH, or the combination of AADC, TH, and GCH. Those studies demonstrated safe gene transfer and robust expression, restoration of DA signaling, and improvements in motor behavior, and have been thoroughly reviewed elsewhere . Key clinical trials assessing dose escalation, safety, and efficacy in PD patients that have moved forward and are currently active include AAV2-hAADC and lentiviral-GCH1-TH-AADC.
AAV2-hAADC
Clinical phase. To date, there have been a total of six Phase 1 open-label clinical studies and one Phase 2 placebo-controlled study utilizing adeno-associated serotype 2 viral vectors for expressing human AADC administered via bilateral intraputaminal infusions , with four of the studies reporting long-term data from the same cohort of patients . At present two studies are active, but not recruiting, VY-AADC01 and VY-AADC02 . A long-term observational extension study for the participants who previously received AAV2-hAADC therapy is enrolling by invitation only .
LV-GCH1-TH-AADC
Recommended Reading: Parkinson’s Disease Stage 1 Symptoms
A Busy Year Ahead For Parkinsons Disease
Parkinsons disease research has ended in numerous dead ends despite substantial efforts over many years. Recently, Biogen and Sanofi scrapped their Parkinsons candidates, cipanemab and venglustat respectively, owing to lack of efficacy, and a disease-modifying therapy has yet to materialise.
But the push to find drugs that help beyond reducing symptoms continues, and Evaluate Vantage has delved into the pipeline of projects in active late-stage clinical trials. This year is shaping up to be crucial for the field, with 10 studies expected to yield data or to complete in 2021.
One target that crops up multiple times is GLP-1 this approach, traditionally employed in type 2 diabetes, is also being tested in Alzheimers. Among other avenues of research, it is hoped that gene therapy could offer a one-time cure for Parkinsons.
Repurposing
Research has suggested that GLP-1 agonists have neuroprotective benefits, and several trials of marketed diabetes drugs, as well as new GLP-1-targeting projects, are under way in Parkinsons. Some of these studies are investigator sponsored, including the most advanced, a UCL-run phase III trial of Astrazenecas Bydureon called Exenatide-PD3.
In the meantime, data are expected from several phase II studies of GLP-1 agonists, including a trial of Novo Nordisk’s Victoza, being run by Cedars-Sinai Medical in collaboration with the Danish company and The Cure Parkinson’s Trust. That study is set to complete in September.
Intraoperative Mri Ensures Targeted Therapy
Dr. Richardson has helped pioneer one such therapeutic infusion: aromatic L-amino acid decarboxylase , an enzyme reduced in Parkinson’s disease. “If you give the brain back AADC, it can make more dopamine in the place where it’s needed,” he says.
For the past ten years, Dr. Richardson has been involved in a multi-institutional effort to deliver gene therapy vectors to the right location. In a paper published in the Journal of Neurology, Neurosurgery and Psychiatry, they describe the evolution of an approach that allows the neurosurgical team to watch vector delivery and make real-time changes as needed:
- The patient is put under general anesthesia
- The team mounts a temporary aiming device on the patient’s skull
- A special infusion cannula developed for this purpose is inserted into the brain, and an infusion pump delivers the vector
- Intraoperative magnetic resonance imaging allows the team to watch the infusate as it is distributed in the brain
- A special software system helps the team align the surgical trajectory and make adjustments if needed
“It’s critical to watch what’s happening you have to adjust the cannula continuously during the infusion based on visual information that the surgeon interprets while watching the infusion distribute in the brain,” Dr. Richardson says.
Examples of adjustments the team can make in real-time include:
- Trajectory and placement of the cannula
- Advancement of the cannula
- Control over where the infusate is going
- Volume and dosing
Recommended Reading: Is Massage Good For Parkinson’s
What Is The Future Of Gene Therapy For Parkinsons Disease
The jury is still out on each of the gene therapy strategies discussed above and each is still under investigation in an active clinical trial, although as of publication of this blog, none are currently recruiting participants. Three of APDAs Centers for Advanced Research are participating in gene therapy trials for Parkinsons disease. Emory University School of Medicine and the University of Alabama at Birmingham School of Medicine are participating in trials of Neurturin. The University of Pittsburgh Medical Center and Emory University School of Medicine are participating in trials of AADC.
Differences In Population Focus For Enzymatic Enhancement Of Da Production Versus Neurotrophic Restoration Of Da System
Selection for enzymatic enhancement
Moderate to severe PD is frequently accompanied by motor fluctuations and unpredictability of response to medications. These symptoms are in part related to complications of long-term use of DAergic medications, but also ongoing neurodegeneration and subsequent loss of key enzymes for DA production . This advanced parkinsonian phenotype is expected to have the greatest benefit with a gene therapy approach to enzymatically reconstitute DA production, as opposed to a de novo or earlier stage PD patient when the remaining endogenous DA machinery is still able to compensate. However, significantly advanced PD is more likely to experience other complications of therapy such as impulse control disorders and DA dysregulation syndrome, which have the potential to worsen with supratherapeutic levels of DA. Enrollment of these individuals should be considered carefully for an unmodifiable gene therapy approach.
Selection for neurotrophic restoration
Don’t Miss: Wolf Parkinsons White Disease Treatment
Who Might Be Useful For Gene Therapy And Are There Any Risks
Researchers believe that gene therapy will be useful for a number of people with Parkinsons, irrespective of whether their condition has been genetically caused. However, as with most treatments, it wont be suitable for everyone. As part of the clinical trial process, scientists will establish who this treatment will suit and those for whom it is not recommended.
As with all treatments, there are some risks. It is thought that there may be side effects within the central nervous system that relate to long-term, high levels of exposure to therapeutic genes, or there may be an immune response to the treatment. Current research efforts are directed at implementing further measures to reduce the probability of these risks, even if they are unlikely.
Restoring Neurotrophic Signaling To The Dopaminergic Network
Though evidence suggests vector-mediated enhancement of DA production is an improvement over current pharmacotherapy, this approach does not address disease progression. Preservation and regeneration of the nigrostriatal pathway is crucial to extending PD patient quality of life and even reversing symptoms of the disease. In addition to DAergic neuron loss, PD patient brains also exhibit significant loss of endogenous neuronal growth factors in affected brain regions . Thus, extensive research on restoring growth factors to encourage sustained DAergic health has been carried out. Preclinical work in NHP models of PD has shown significant promise from intraparenchymal infusions of growth factor-based protein or gene therapy to stabilize and even restore DAergic signaling via sprouting of remaining DA projections, thereby ultimately improving motor symptoms .
AAV2-GDNF
Clinical phase. AAV2-GDNF has been used in one Phase 1 dose-escalation study that is nearing completion , and an actively recruiting Phase 1b study utilizing MRI-guided bilateral putaminal delivery of the study drug .
Safety. AAV2-GDNF administration has so far demonstrated a robust safety profile. Of the 13 participants in the Phase 1 study, only one SAE was reported related to the surgical procedure, which was a scalp wound dehiscence requiring debridement. No other SAEs were attributed to either the surgical procedure or the AAV2-GDNF drug.
Recommended Reading: How To Improve Walking With Parkinson’s