What Are The Main Issues With Current Treatments
Long-term use of dopamimetic agents, in combination with continued dopaminergic denervation, can generate dyskinesia. Indeed, while dyskinesia are mainly associated with functional alterations within the basal ganglia pathways related to prolonged exposure to L-DOPA, dopamine agonists and DBS can also cause the appearance of dyskinesia . The exact mechanism underlying dopamine agonist- or DBS-induced dyskinesia is still under investigation, but it is believed to stem from maladaptive mechanisms related to dopaminergic and glutamatergic systems . Patients receiving intra-striatal dopaminergic neural grafts can also experience dyskinesia, also without the presence of exogenous dopaminergic agents , possibly due to inappropriate responses to dopamine release by grafted neurons .
If Levodopa Causes Dyskinesia Then Why Should I Take It
At present, treatment with levodopa is the most effective way to relieve tremor, stiffness, and slow movement associated with Parkinsons. In the early stage of Parkinsons, levodopa may not be necessary and there are other medications available to treat this stage of the disease. However, as the disease progresses and symptoms begin to interfere with daily living, your doctor will prescribe levodopa.
- It typically doesnt develop immediately Its important to note that there is usually a time lag of roughly 4 to 10 years from the start of treatment with levodopa to when dyskinesia emerges, and its severity will vary among different individuals.
- Younger people are at a greater risk People who get Parkinsons in their later years may not show signs of dyskinesia or may have only mild symptoms within their lifetime. Being diagnosed with Parkinsons at a younger age is associated with a greater chance of developing dyskinesia.
- As with every aspect of Parkinsons, there is variability in dyskinesias Some do not develop dyskinesias at all. For those who do get them, not all experience them the same. Dyskinesia in its milder form may not be bothersome, and the mobility afforded by taking levodopa may be preferable to the immobility associated with not taking levodopa. People with Parkinsons must weigh the benefits from using levodopa versus the impact of dyskinesia on their quality of life.
Dystonia Vs Dyskinesia In Parkinson’s Disease
Dystonia and dyskinesia are movement problems that commonly occur in Parkinsons disease . You may experience one or both of them, particularly in late-stage PD. Dystonia is muscle stiffening caused by PD, while dyskinesia is a type of muscle twisting caused by some PD medications.
Dystonia and dyskinesia can both cause distress, and they are distinguished from each other based on their visible features. They can be managed with medication or surgery, typically with a moderate improvement of symptoms.
PD is characterized by four primary symptoms:
- Postural instability
While they can fluctuate in severity, the primary symptoms of PD tend to be present most of the time.
Dystonia and dyskinesia are recurrent, abrupt, and short-lived muscle movements. Not everyone who has PD experiences dystonia and dyskinesia. If they do, the symptoms they experience can be telling.
-
Affects large muscle groups
-
Smooth, repetitive movement often described as a rolling or writing motion
-
Can begin suddenly and stop after several minutes
-
Not typically painful
-
More likely to occur when PD medication effects are at their peak
For example, dystonia can cause your toes to curl, making it difficult to walk. Or it may manifest primarily in your neck muscles, causing your head to turn painfully to one side.
With dyskinesia, you may experience a snakelike twisting of your arm or movements of your head and neck that appear like dancing in slow motion.
Don’t Miss: Can Parkinson’s Start In The Legs
Tweak The Timing Of Your Medication
The timing of medication is also a consideration, because of wearing off phenomenon, in which some patients feel that the effects of the medication end about four hours after a dose. Your doctor may decide to split your daily medication into smaller, more frequent doses. Doing so may deliver a steadier amount of medication to the body, according to a 2016 research review in the European Medical Journal.
Your doctor may also suggest a switch to extended-release pills, which work in a similar manner. The downside to these formulations, however, is that they tend to require more of the drug to achieve the same result.
Pharmacologic Strategies To Directly Address The Incidence Of Dyskinesias
Restoration of striatal dopaminergic stimulation is the goal in the treatment of parkinsonian motor symptoms. Levodopa provides the greatest benefit for treating parkinsonian motor dysfunction, but because its use is associated with the development of motor complications, one of the great unmet needs for the treatment of PD is a medication that will match the efficacy of levodopa but not cause motor complications. Until such a medication is available, it is useful to identify treatment strategies that can provide adequate efficacy while minimizing motor complications.
The short half-life of levodopa and the resultant pulsatile dopaminergic stimulation appear at least in part to be responsible for the development of motor complications . Therefore, CDS may delay the onset of dyskinesias in early disease and alleviate dyskinesias in advanced disease.
Also Check: Parkinson’s Disease Hospice Criteria
Prevention And Management Issues Of Levodopa
Theoretical consideration that levodopa may accelerate neuronal degeneration due to oxidative stress led to levodopa-sparing therapy. In a 5-year study, cumulative incidence of dyskinesia was 20% in the ropinirole group and 45% in the levodopa group. In another study, 54% of patients in the levodopa group and 25% of patients in the pramipexole group had dyskinesias. However, initial treatment with levodopa had lower incidences of freezing, somnolence, and edema. CALM-PD trial showed significant less number of PD patients with dyskinesia who took pramipexole in comparison to levodopa . However, the Movement Disorder Society evidence-based review panel concluded that there was insufficient evidence to support the use of pramipexole extended release to delay or treat LID. They also concluded that ropinirole and ropinirole extended-release preparations are effective in prevention of dyskinesia, but there is insufficient evidence for their use in treatment of dyskinesia. Rotigotine transdermal patch has been used to manage PD patients with dyskinesia, but data on using for prevention of dyskinesia are insufficient. In addition, the evidence-based review concluded that rasagiline, a long-acting monoamine oxidase B inhibitor, is insufficient for preventing and treating LID.
Clinical Features And Classification Of Lid
LID are clinically heterogeneous. They commonly present as chorea or choreoathetosis, though myoclonus, akathasia, ballism and other forms of abnormal movements have also been described. LID generally first appear on the side worst affected by Parkinsons disease and in legs before arms. This could be related to an early dopaminergic loss in the dorsolateral striatum, the region corresponding somatotopically to the foot area.
Chorea refers to involuntary, rapid, irregular, purposeless, and unsustained movements that seem to flow from one body part to another. The severity of these movements can vary from occasional abnormal movements that are absent at rest and provoked only during active movementfor example, walking or talking to violent large amplitude flinging and flailing arm movementsthe ballism. Often, there are superimposed writhing athetoid movementschoreoathetosis. Dyskinesias may predominantly affect particular body partsfor example, torso, head and neck, limbsor speech or respiratory muscles.
Dystonia is the second most common form of LID presenting as sustained muscle contractions. It occurs either in pure form or in combination with the chorea, in the latter case manifesting as twisting of the leg on walking or the arm being pulled behind the back. Dystonia accounts for greater disability than chorea. Off time dystonias are usually painful.
You May Like: What To Expect As Parkinson’s Progresses
Medications And Supplements Used To Treat Tardive Dyskinesia
A number of medications and supplements have been identified that ameliorate TD symptoms.
Cholingergic Agents.
Cholinergic agents are used as muscle stimulants to diagnose myasthenia gravis and to treat glaucoma. These agents can also improve the Parkinsonian features of TD. Donepezil, a reversible acetylcholinesterase inhibitor, is currently the only cholinergic medication that has shown benefit against TD. Overall, however, cholinergic agents are not a widely accepted treatment for TD as sufficient evidence is lacking to suggest they are more helpful than other treatments.
Clozapine, Quetiapine, Olanzapine, and Apomorphine.
Clozapine, a serotonin and dopamine receptor antagonist, is an atypical APD used to treat schizophrenia. Clozapine is the best current medication recommended for patients who require antipsychotics and simultaneously have TD, as clozapine has been reported to reverse TD symptoms., Clozapine has been linked to TD however, the incidence is much lower compared to other atypical APDs. Drugs with similar mechanisms of action such as quetiapine, a weak striatal dopamine antagonist, and olanzapine, a dopamine and serotonin receptor antagonist, have also been shown to be effective in ameliorating TD symptoms. Apomorphine, a dopamine receptor antagonist, can be given in conjunction with L-DOPA to decrease dyskinesias.
Tetrabenazine Analogs.
Rating Scales For Dyskinesia
Different scales and instruments have been used to provide objective assessment of LID and its impact on overall quality of life. UPDRS is helpful in assessment of different aspects of dyskinesia, but it does not include the anatomical distribution of dyskinesia in different body parts. There are other scales used to assess LID, including the Rush Dyskinesia Rating Scale , Unified Dyskinesia Rating Scale, and Clinical Dyskinesia Rating Scale . There are different instruments to measure quality of life, including 39-item Parkinson’s Disease Questionnaire and Parkinson’s Disease Quality of Life Scale. Patients self-evaluation diaries such as Hauser diary have been used to know the effect of drugs used to treat LID, but the compliance to diary completion and accuracy is extremely challenging. Several quantitative instrumental techniques have been developed to quantify dyskinesia including wearing devices, accelerometers, and position transducers.
Don’t Miss: How Do You Get Parkinson’s Disease
Fewer Symptoms Better Quality Of Life After Dbs
Before the procedure, 11 patients worked full time and four part time the remaining 31 patients did not work . After DBS, at the end of the 12-month follow-up period, 15 patients were working, with nine in full-time and six in part-time jobs.
Regarding mutations, seven patients in the group analyzed had known Parkinsons disease-causing mutations. Results showed no significant differences in motor symptoms after DBS between patients with and without disease-associated mutations.
In terms of safety, severe side effects associated with DBS included postoperative confusion , wound healing issues , and intracranial bleeding around one electrode .
Overall, the team concluded that the present study-results demonstrate that EOPD patients with and without known genetic background benefit from STN-DBS with significant improvement in motor as well as non-motor symptoms.
Furthermore, patients experienced significant 30% improvements of measured by the PDQ-39, the researchers added.
Nonetheless, the team recognized that a better understanding of the genetic background and associated clinical features might have an impact on decision making in DBS and the eventual individual outcome.
In fact, the team started a patient registry. Its aim, they noted, is to to gain knowledge on patients progression and long-term outcome that might enable clinicians to improve the counseling of EOPD-patients.
Basic Pathophysiology Of L
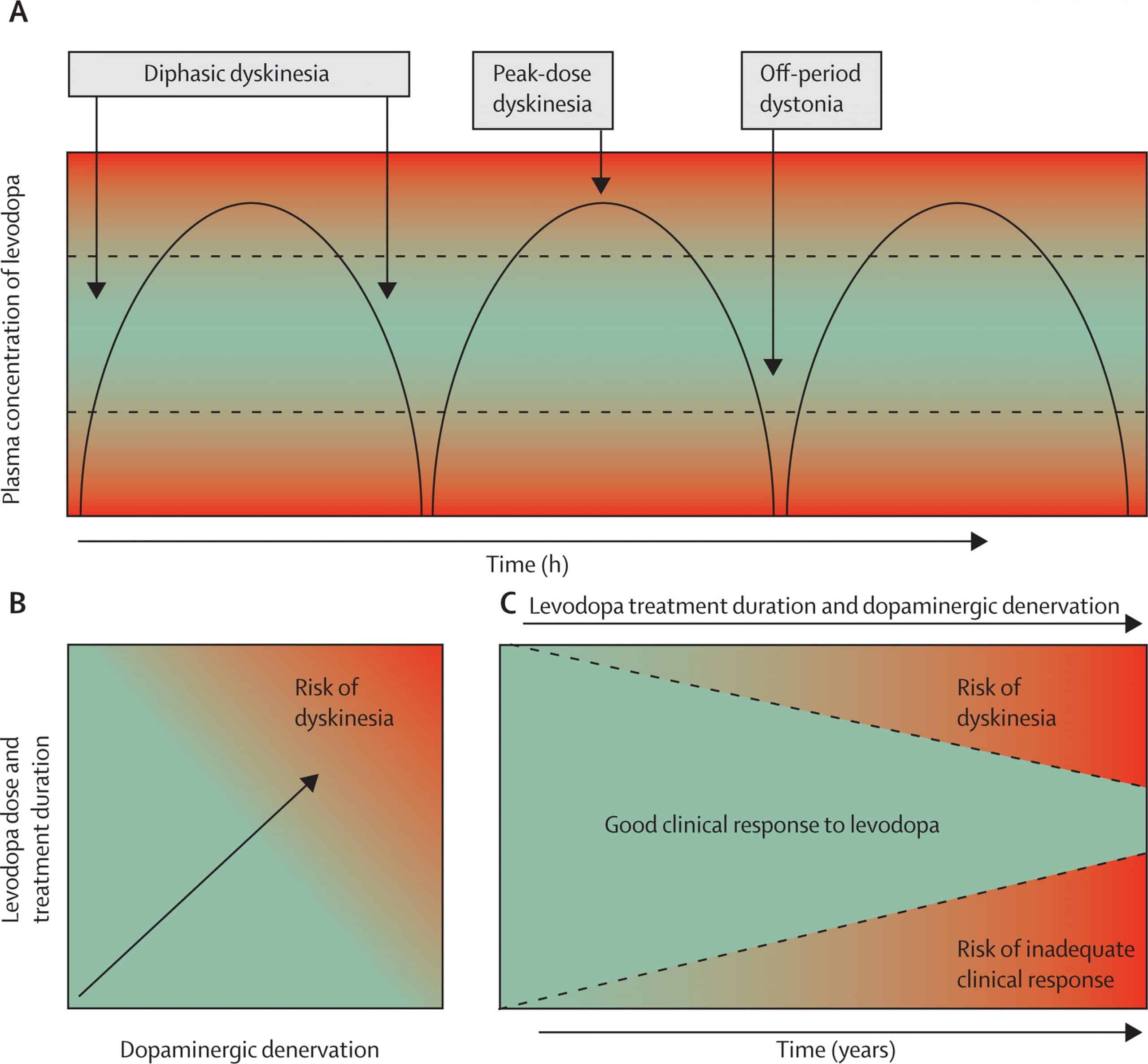
The mechanism of LID is not clearly understood. However, continuous research has found that continued damage of nigral dopaminergic neurons produces deformities in the connection of the motor cortex and the striatum and makes a functional disturbance in basal ganglia, which can lead to the generation of involuntary abnormal movements, that is, LID . The quantity and period of drug exposure play a crucial role in the development of dyskinesia. Along with that, the severity of LID also depends on the extent of neurodegeneration. PD patients and MPTP-treated primates having a higher degree of dopaminergic neuron degeneration show dyskinetic symptoms after the administration of L-DOPA. In general, rising and falling of the plasma L-DOPA level is the main reason for dyskinesia and motor defect .
Along with the advancement of the disease, the equal dosage of L-DOPA, which is generally required for alleviation of PD symptoms, causes dyskinesia. The main cause for this altered response pattern is not clear however, the literature suggests that disturbance in between pre- and postsynaptic nigrostriatal DA transmission leads to motor complications. A schematic diagram describing major signaling abnormalities associated with LID in PD patients has been illustrated in Figure 1.
Don’t Miss: Do All Parkinson’s Patients Get Dementia
Reduction Of Levodopa Doses
The peakdose LID almost always respond to a dose reduction. However, this results in worsening of parkinsonism and increasing off periods. The strategy of temporary withdrawal of levodopa is not used as it is often associated with significant worsening of Parkinsons disease and dyskinesias are only slightly reduced for a short period of time.39 The frequent and small doses often fail to achieve desired results. Patients prefer mobility associated with dyskinesias to immobility with no dyskinesias.
Delivering Levodopa In A More Continuous Fashion

Optimizing levodopa therapy by adding an agent that can prolong its half-life and deliver it in a less pulsatile manner is another promising way to achieve CDS. Levodopa is metabolized peripherally by aromatic amino acid decarboxylase and COMT. The combination of levodopa/carbidopa plus the COMT inhibitor entacapone can reduce the peripheral conversion of levodopa and extend the levodopa half-life to 2.5 h . In MPTP-treated monkeys, Jenner showed that coadministration of the same dose of levodopa 4 times a day with entacapone improved parkinsonian motor response and caused less dyskinesia than treatment with levodopa alone. Peak dose dyskinesia scores and dyskinesia duration were decreased . Rat studies showed similar results , supporting the theory that reducing pulsatile delivery of levodopa leads to fewer motor complications. Clinical trials are now under way in which patients in need of levodopa therapy are randomly assigned to treatment with levodopa and carbidopa, or levodopa, carbidopa and entacapone. The aim is to determine whether the introduction of entacapone when levodopa and carbidopa are first administered will lower the rate of dyskinesia onset.
Also Check: Surgery For Parkinson’s Tremor
The Difference Between Off And Parkinsons Dyskinesia
Although OFF times and dyskinesia are common aspects of living with Parkinsons and are both linked to Parkinsons medications, they are not as alike as you might guess. In this post, we highlight the differences between OFF and dyskinesia and strategies to manage them.
Dyskinesia is an uncontrolled, involuntary muscle movement that, unlike tremor, is irregular in motion. In people with Parkinsons, it is most often associated with long-term use of levodopa or other Parkinsons medications that increase dopamine levels in the brain. While dyskinesia is a minor annoyance for some, for others, it can interfere with daily life and compromise gait and balance. It can also lead to embarrassment or unwanted attention during everyday social activities, such as dining in public or grocery shopping.
In similar ways, feeling OFF can cause someone to experience gait and balance issues, avoid public gatherings and self-isolate, and experience a lowered quality of life. OFF, however, is much more nuanced than dyskinesia. How you feel when youre OFFwhen your medications arent working optimally, and your Parkinsons symptoms returncan be difficult to describe, ever-changing, and completely unique to you. While dyskinesia can also look different depending on the person living with it, it presents in common ways and typically is more easily recognized by friends and family.
Read Also: Can Parkinsons Be Reversed With Diet
Altered Dopaminergic Pathways And Receptors
Three signaling pathways, PKA/DARPP-32, ERK, and mTORC1, were identified, which exert significant involvement in the pathophysiology of LID. All these pathways are interrelated and are activated by a common intercellular cascade triggered in nigrostriatal SPNs expressing D1 receptors. Chronic L-DOPA administration activates all of them, which alters the striatal synaptic plasticity. DARPP-32 signaling was observed to regulate ERK and mTORC1 pathways .
Recent studies have demonstrated that D1 receptor activation leads to Shp-2 activation, which leads to activation of ERK1/2 and mTOR phosphorylation. This, in turn, induces the LID expression. This effect can easily be reversed by using a D1 receptor antagonist, which downregulates the end product of the D1R/Shp-2 pathway, p-mTOR, and p-ERK1/2 . Rapamycin was observed to be selectively affecting the mTORC1 pathway, without affecting DARPP-32 signaling, ERK pathway, and AMPA, NMDA expression .
An increased expression of D3 receptors was reported in animals with severe LID. An associated increase in the expression of GABA release was also noticed. D3 receptors can also perform the D1 receptor modulation action via affecting the ERK pathway. Upon deletion of the D3 receptor, levels of FosB, ERK, and H3 activities decreased along with the alleviation of LID symptoms, with no effect on L-DOPA treatment . A lack of D5 receptors is also associated with increased LID severity and decreased response to the L-DOPA symptoms .
Also Check: Parkinson’s Masked Face Images
Prevalence Of Motor Complications By Disease Duration Treatment Duration And Hoehn And Yahr Stage
The rates of occurrence of motor fluctuations and dyskinesias in levodopa-treated patients with a disease duration of 5 years or less were 14 and 7%, respectively. After a disease duration of 69 years they had occurred in 39 and 18%, respectively, and after 10 or more years of disease they had occurred in 67 and 57%. These rates were lower if the overall sample including patients not on levodopa was considered, and greater when only those who had a good or excellent response to levodopa, or those with levodopa doses of > 300 mg were considered .
After a treatment duration of 5 years or less, 21 and 13% of patients on levodopa had developed fluctuations and dyskinesias, respectively, after 69 years of treatment 56 and 36%, and after 10 or more years all patients had developed dyskinesias and motor fluctuations.
Motor complications had not occurred in patients on levodopa with a Hoehn and Yahr stage of 1 or 1.5 , but 42% of those in stages 2 or 2.5 had motor fluctuations and 29% had dyskinesias. In stage 3 patients, motor fluctuations were present in 50% and dyskinesias in 43%, and in patients in Hoehn and Yahr stages 4 or 5 they were present in 71% and 60%, respectively.
Acknowledgements And Conflict Of Interest Disclosure
RM acknowledges grants from the Spanish Ministries de Economía y Competitividad and of Sanidad Política Social e Igualdad, ISCIII: BFU2010-20664, PNSD, CIBERNED ref. CB06/05/0055 and Comunidad de Madrid ref. S2011/BMD-2336, JRGM is supported by ICyTDF México MTH acknowledges the support by CIBERNED CB05/05/505, SAF2007-062262 and FIS PI10-02827. RH and KC were supported by the German Bundesministerium für Bildung und Forschung, Grant 01GN1006B. NS gratefully acknowledges Sardinia Regional Government for financial support . The authors have no conflicts of interest to declare.
All experiments were conducted in compliance with the ARRIVE guidelines.
You May Like: Signs Of Parkinsons Disease
Recommended Reading: Motor Symptoms Of Parkinson’s Disease