Diagnosis And Pharmacological Management Of Parkinsons Disease: Summary Of Sign Guidelines
Rationale For Early Levodopa Use
Table 1 Comparison of changes in UPDRS scores between levodopa therapy and dopamine agonistsFull size table
However, levodopas superiority in alleviating PD symptoms has been dampened in some patients with a higher propensity to developing medication-induced complications. Head-to-head comparisons between levodopa and dopamine agonists over a 2- to 5-year period uniformly showed a higher frequency, and at a sooner timepoint, of dopaminergic complications, particularly dyskinesia, among early PD patients randomized to levodopa . Nonetheless, while the time-to-onset of dyskinesia was shorter with levodopa therapy , this difference becomes non-significant at 3 years of follow-up, although the severity of dyskinesias remains lower in patients taking dopamine agonists, regardless of levodopa augmentation . On the other hand, the time-to-onset and severity of motor fluctuations did not differ between levodopa and dopamine agonists .
Editorial Note On The Review Process
F1000 Faculty Reviews are commissioned from members of the prestigiousF1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions .
The referees who approved this article are:
-
Fredric P. Manfredsson, Parkinsons Disease Research Unit, Department of Neurobiology, Barrow Neurological Institute, Phoenix, Arizona, USA
No competing interests were disclosed.
-
Tipu Z. Aziz, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, UK
No competing interests were disclosed.
Don’t Miss: Sam Waterston Tremor
Getting Medication On Time
Parkinson’s patients who do not get the correct medicine at the right time when they are in hospital, sometimes cannot talk or walk. The health of a majority deteriorated due to unsatisfactory medication management when they are in hospital. Parkinson’s UK believes the NHS could save up to £10m a year and improve the care of Parkinson’s patients if mandatory training is introduced for all hospital staff.
Parkinson UK found:
- “Nearly two thirds of people who have Parkinsons dont always get their medication on time in hospital.”
- “More than three quarters of people with Parkinsons that we asked reported that their health deteriorated as a result of poor medication management in hospital.”
- “Only 21% of respondents told us they got their medication on time without having to remind hospital staff.”
Iidopamine Receptor Supersensitivity In Parkinson’s Disease
Treatment of Parkinson’s disease with L-DOPA remains the primary therapy. While a very effective therapy, long-term treatment invariably leads to the development of dyskinesias . We have proposed that L-DOPA-induced dyskinesia in the treatment of Parkinson’s disease results from an aberrant switch in the linkage of the D1 receptor to signal transduction systems that activate the protein kinase, extracellular signal-regulated protein kinase . As discussed, dopamine depletion of the striatum results in opposite effects on the function of D2-indirect and D1-direct pathway neurons evidenced by changes in gene expression . While, either L-DOPA or selective D2 and D1 receptor agonist treatments reverse some of the gene expression changes, the response of D1 receptor-expressing direct pathway neurons is supersensitive to these treatments, which is evident by the induction of a large number of so called immediate-early genes .
E. Cubo, CG. Goetz, in, 2014
Read Also: Parkinson Bicycle Cleveland Clinic
Aan Releases Recommendations On Treatment Of Parkinsons Disease
Am Fam Physician. 2007 Mar 15 75:922-924.
-
Guideline source: American Academy of Neurology
-
Literature search described? Yes
-
Evidence rating system used? Yes
-
Available at:
Parkinsons disease is the second most common neurodegenerative disease and is characterized by bradykinesia tremor at rest rigidity and abnormalities of balance, posture, and gait. Its etiology remains unknown in most patients. Recommendations from the Quality Standards Subcommittee of the American Academy of Neurology discuss the following aspects of this condition in a collection of articles in the April 2006 issue of Neurology: diagnosis and prognosis neuroprotective strategies and alternative therapies treatment and evaluation and treatment of depression, psychosis, and dementia in patients with Parkinsons disease.
Treatment Of Motor Fluctuations
A number of strategies can reduce motor fluctuations in patients with PD. Optimising the amount of levodopa delivered to the brain is the main approach and can be achieved by increasing the levodopa dose, adjusting the timing of administration and/or adding adjunctive agents., Administering levodopa with a low protein meal or empty stomach, if tolerated, can improve absorption. Smaller, more frequent dosing may also help. Changing to a controlled-release formulation could theoretically improve fluctuations however, studies have shown no difference in symptoms compared to immediate-release preparations.
Adjunctive agents such as dopamine agonists, catechol-o-methyltransferase inhibitors and monoamine oxidase-B inhibitors have been shown to improve fluctuations. Direct head-to-head studies comparing these medications are lacking however, a Cochrane review involving 44 randomised controlled trials suggested dopamine agonists were most effective in reducing âoffâ time . In older people, choice of adjunctive agent should be based on factors including comorbidities, adverse effects and patient preference.
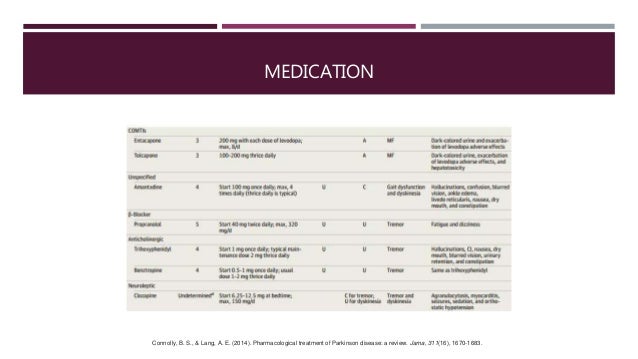
Dopamine Agonists
COMT Inhibitors
MAO-B Inhibitors
Don’t Miss: Zhichan Capsule
Pathophysiology And Presenting Features
Classic presenting features of PD include motor symptoms, such as bradykinesia, rigidity, rest tremor and postural instability. However, non-motor symptoms, such as depression, cognitive impairment, pain and autonomic disturbances, are also often present and they can severely affect a patients quality of life. There are several information sheets available for patients that cover the management of multiple common types of pain in PD.
The motor symptoms are largely caused by the progressive loss of dopaminergic neurons in the substantia nigra compacta, which ultimately reduces dopaminergic input to the striatum and other brain regions. Compensatory mechanisms in the brain are so effective that the clinical symptoms of PD may only develop when around 80% of dopaminergic neurons have degenerated. By contrast, the Braak theory of PD suggests that the disease process starts in the olfactory bulb and lower part of the medulla, and it is not until stage 3 that the substantia nigra becomes involved in the process. There is also direct evidence of Parkinson pathology being spread from the gastrointestinal tract to the brain in rodents. There are therapeutic implications of gut involvement it is known that swallowing and the stomach are the two main problems of PD therapy and lead to the use of non-oral therapies.
Basics Of Parkinsons Disease
Parkinsons disease , or paralysis agitans, is a common neurodegenerative condition, which typically develops between the ages of 55 and 65 years. This disease was first named and described by James Parkinson in 1817. The progression of this disease is gradual and prolonged. It has a plausible familial incidence, although the estimates of these occurrences are low and usually sporadic. This disease is organized into two classifications: genetic and sporadic. Genetic PD follows Mendelian inheritance. Sporadic PD, which accounts for about 90% of all Parkinsons cases, is a more complex category in which the pathogenic mechanisms that underlie it are not yet fully understood. Nonetheless, it is known that the byzantine interactions of genetic and environmental influences play roles in the determination of sporadic PD. Several subtypes of PD exist. Each has its own set of causative factors and susceptibilities, pathology, and treatment courses. General risk factors, symptoms, and pathology will be discussed first, before addressing some of the subtypes.
Recommended Reading: Parkinson’s Hallucinations Commercial
Motor Fluctuations And Dyskinesia
For the treatment of motor features of tremor, bradykinesia, and rigidity associated with Parkinsons disease, dopaminergic therapies are initially effective however, motor fluctuations eventually complicate therapy and can cause significant disability and impair quality of life. Sustained-release carbidopa/levodopa and bromocriptine have not been found to reduce off time.
Risk factors for motor complications include disease severity, younger age at onset of Parkinsons disease, high levodopa dosage, and longer disease duration. The motor fluctuations usually are addressed with levodopa adjustments as well as adjunctive medications or surgery as discussed below.
Read Also: What Tests Are Done To Diagnose Parkinsons
Anticholinergics For Early On
The first pharmacological agents used in PD therapy were anticholinergic drugs. They reduce the activity of acetylcholine by acting as antagonists at choline receptors, hoping to restore the balance between dopamine and acetylcholine levels that was disturbed by PD. These drugs have largely been replaced by L-DOPA and other centrally acting dopaminergic agonists, but they still remain available for use in the treatment of PD. Benztropine, biperiden, diphenhydramine, ethopropazine, orphenadrine, procyclidine, and trihexyphenidyl are included in this therapeutic class of drugs, though there is little pharmacokinetic information available on them because of their low plasma drug concentrations. Typically, anticholinergic drugs have a greater role in tremor-predominant PD and can be a monotherapy in early stages, but are usually done in adjunct with L-DOPA or other prescribed medications.
You May Like: Yopd Life Expectancy
Manual Therapy And Exercise
Chiropractic manipulation, osteopathic manipulation, and Trager therapy have been suggested to benefit patients with Parkinsons disease. No studies exist, however, to refute or confirm this position. The Alexander technique has shown some benefit and patient improvement has been noted in some studies.
Standard physical therapy, as well as occupational therapy, did result in improved functional outcomes, but the benefit was small and was not sustained when the exercise therapy stopped.
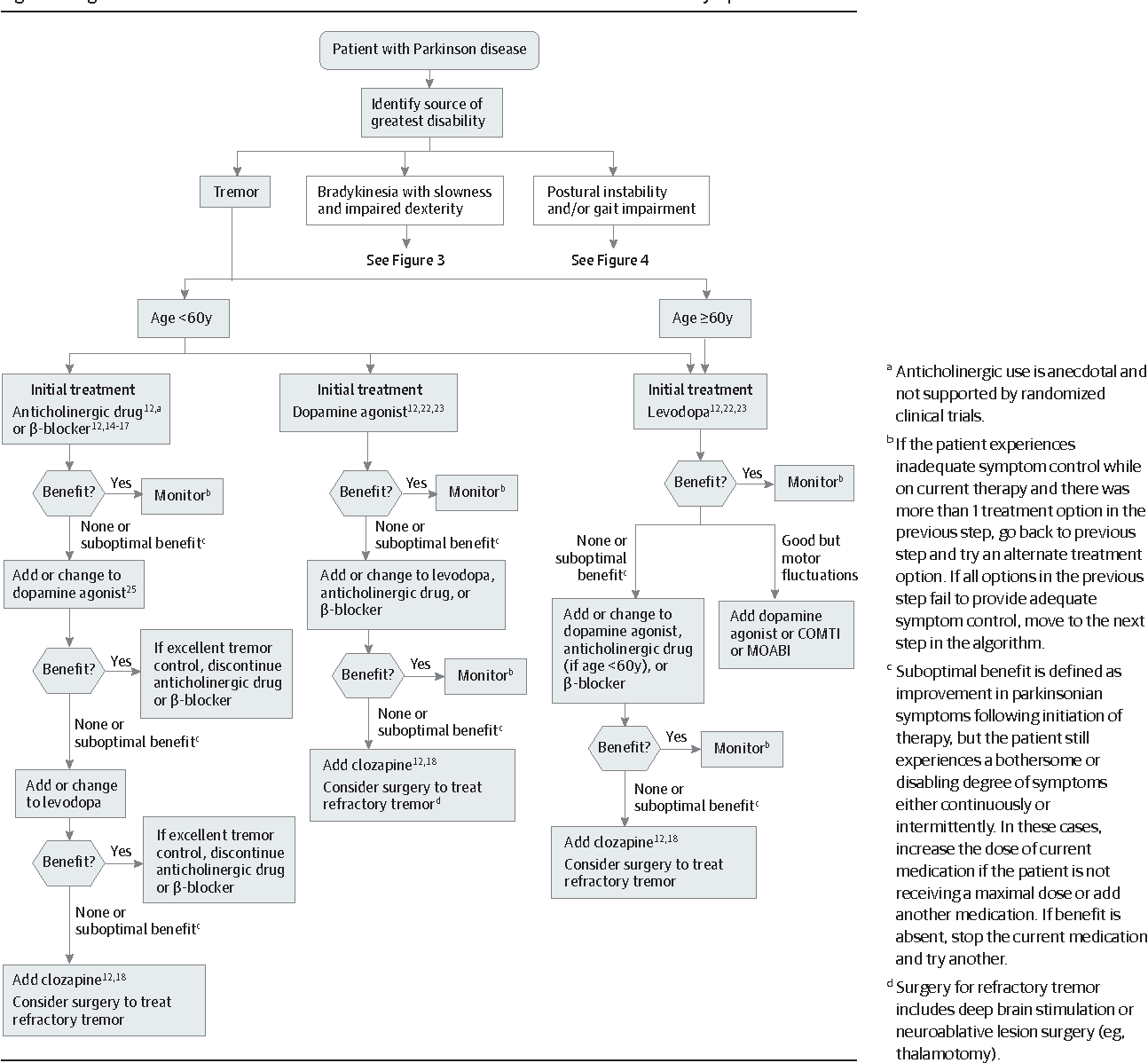
When To Start Treatment
Deciding when to start drug therapy for Parkinsons disease should be individually tailored to a patients symptoms, circumstances and comorbidities. Treatment is indicated when symptoms impact on quality of life. When treatment is needed there is no evidence to support undue delay because of concerns about levodopa toxicity or the development of treatment resistance.3 The aim is to control symptoms and maintain an on state.
Some drugs with good symptomatic benefit are speculated to have a role in neuroprotection and some specialists advocate their use from the time of diagnosis.4 Delayed start trials have been used to try and differentiate symptomatic from disease-modifying effects. A recent delayed start study of rasagiline, a monoamine oxidase B inhibitor, in treatment-naïve patients with mild Parkinsons disease showed a small benefit in the low-dose treatment group. This was not seen with the 2 mg dose and a clear explanation for this has not been established.5 Further studies are needed before such treatments are considered truly disease modifying. Until a drug is unequivocally proven to slow disease progression, the time to commence treatment will remain contentious.
Dont Miss: On Off Phenomenon In Parkinsons Disease
You May Like: What Foods Should Be Avoided When Taking Levodopa
Levodopa: The Most Effective Drug For Treating Parkinsons
Levodopa, also known as L-DOPA, has long been, and continues to be, the most effective drug in treating Parkinsons disease symptoms. Most people with Parkinsons disease will take this drug at some point. There are side effects that can occur with Levodopa including nausea, fatigue and orthostatic hypotension. Often these side effects can be successfully treated so that Levodopa can be tolerated better. In addition, as the disease progresses and the brain has less ability to produce and process dopamine, dyskinesias, or involuntary movements can develop from Levodopa.
Monoamine Oxidase B Inhibitors
Other PD medications work by inhibiting the enzymes involved in dopamine metabolism, which preserves the levels of endogenous dopamine. One such class is the MAO-B inhibitors. As is discussed above, MAO-B is one of the main enzymes involved in the breakdown of dopamine, and reducing the activity of this enzyme therefore results in increased dopaminergic activity within the striatum, mediated by endogenous dopamine . Their use relieves motor symptoms in PD patients, and as with dopamine agonists they may be used as an initial treatment option, to delay the need for levodopa therapy, to reduce the risk of levodopa-induced motor complications . While they are sometimes sufficient for control of symptoms in early disease, most patients ultimately require levodopa-based treatment. MAO-B inhibitors may also be used in combination with levodopa-based preparations, to allow for a reduction in the levodopa dose.
You May Like: Pfnca Wellness Programs
Evidence For Using Dopamine Agonist
As mentioned above, the caveat of long-term levodopa therapy consists of development of motor fluctuations, dyskinesias, and wearing off phenomenon. Although, as mentioned earlier, these complications are likely caused by several factors including drug pharmacokinetics, disease duration, severity, and duration of levodopa therapy, these are seen more often among levodopa responders . This has led to investigations for levodopa-sparing strategies, primarily utilizing dopamine agonists for early monotherapy . Pramipexole, rotigotine, and ropinirole are non-ergot dopamine agonists which were studied to be effective treatments for early PD . Other dopamine agonists, such as bromocriptine, pergolide, and cabergoline, were previously utilized as treatment but were discontinued, or largely underutilized, due to their serious adverse effects .
Another dopamine agonist, rotigotine, a transdermal patch with an optimal dose of 2 to 16 mg/24 h, was shown to be tolerated up to 6 years . A study on early PD patients comparing rotigotine to placebo demonstrated 9.0 to 13.5 mg as the minimum effective dose range, with a doseresponse improvement in UPDRS motor scores .
Common Drugs For Parkinsons Disease
Levodopa and carbidopa . Levodopa is the most commonly prescribed medicine for Parkinsonâs. Itâs also the best at controlling the symptoms of the condition, particularly slow movements and stiff, rigid body parts.
Levodopa works when your brain cells change it into dopamine. Thatâs a chemical the brain uses to send signals that help you move your body. People with Parkinsonâs donât have enough dopamine in their brains to control their movements.
Sinemet is a mix of levodopa and another drug called carbidopa. Carbidopa makes the levodopa work better, so you can take less of it. That prevents many common side effects of levodopa, such as nausea, vomiting, and irregular heart rhythms.
Sinemet has the fewest short-term side effects, compared with other Parkinsonâs medications. But it does raise your odds for some long-term problems, such as involuntary movements. An inhalable powder form of levodopa and the tablet istradefylline have been approved for those experiencing OFF periods, OFF periods can happen when Parkinsonâs symptoms return during periods between scheduled doses of levodopa/carbidopa.
People who take levodopa for 3-5 years may eventually have restlessness, confusion, or unusual movements within a few hours of taking the medicine. Changes in the amount or timing of your dose will usually prevent these side effects.
Dopamine agonists. These drugs act like dopamine in the brain. They include pramipexole , rotigotine , and ropinirole , .
Don’t Miss: On And Off Phenomenon
Systematic Review: Efficacy And Safety Of Medical Marijuana In Selected Neurologic Disorders
Current systematic review. Endorsed by the American Autonomic Society, the American Epilepsy Society, the Consortium of Multiple Sclerosis Centers, the International Organization of Multiple Sclerosis Nurses, and the International Rett Syndrome Foundation. Reaffirmed January 21, 2014, and January 11, 2020.
Also Check: Latest Cure For Parkinsons Disease
Diagnosis Of Parkinsons Disease
The diagnosis of PD is clinical and requires bradykinesia, defined as slowness of movement and decrement in amplitude or speed, usually assessed using finger tapping, foot tapping or pronationsupination hand movements. In addition, rest tremor or rigidity is required to confirm a parkinsonian syndrome. Tremor was absent at presentation in 30% in one series of pathologically proven PD. Patients with suspected PD should be referred quickly and untreated to a specialist in movement disorders for evaluation. Key points for discussion at diagnosis include the need to inform vehicle licensing agencies and insurers, signposting to written or web-based information on newly diagnosed PD, and provision of contact details for the local PD nurse specialist .
Current International Parkinson and Movement Disorder Society diagnostic criteria for Parkinsons disease adapted from Postuma RB, Berg D, Stern M et al. MDS clinical diagnostic criteria for Parkinsons disease. Mov Disord 2015 30:1591601. At least two supportive criteria and no red flags required for a diagnosis of clinically established Parkinsons disease. Conditions in italics should be considered if the corresponding exclusion criteria or red flags are present.
Recommended Reading: How To Prevent Getting Parkinsons Disease
Recommended Reading: Voice Amplifiers For Parkinson’s
Canadian Guidelines On Pd
The Canadian Guidelines on PD were developed to address the need to provide health care providers who treat individuals with PD with a tool to guide the best management of the disease. The CGPD are the result of a joint effort by specialists in the treatment of movement disorders, family physicians, allied health professionals, individuals diagnosed with PD and patient advocacy groups such as Parkinson Society Canada. The aim of the CGPD is to improve the care for all Canadians who have been diagnosed with PD through the application of
-
best published evidence,
-
patient involvement in the choice of treatment and informed decision-making, and
-
relevance to the Canadian health care system.
A comprehensive description of the guideline development process is provided with the publication of the CGPD in the Canadian Journal of Neurological Sciences. Since there are other current, high-quality guidelines available for the management of PD, the goal of the CGPD was to review these guidelines to select the recommendations most relevant to the Canadian health care provider.
Pathophysiology And Risk Factors

The loss of dopaminergic neurons in the substantia nigra and presence of alpha-synuclein clumps known as Lewy bodies and Lewy Neurites are the pathological hallmarks of PD. The resultant deficiency in dopamine and relative cholinergic excess lead to motor symptoms. When symptoms present, it is estimated that 60â70% of neurons are already lost. Other neurotransmitters in nondopaminergic areas of the brain are also affected, which likely accounts for the non-motor features.,
Age is the leading risk-factor, with most diagnoses occurring after age 65., Gender ratios vary between studies but overall there is a slight male predominance. Some studies have suggested a genetic link, but this only accounts for 5â10% of PD cases, typically with younger onset., Environmental factors such as pesticide and heavy metal exposure are also suspected, but are not conclusive risk-factors. Caffeine and cigarette smoking are associated with less risk of developing PD.,
Don’t Miss: Similar To Parkinsons