Postsynaptic Dopamine D2 Receptor
In drug-naïve PD patients compared to controls, binding potential for the G-protein-coupled dopamine D2 receptors measured using 11C-raclopride PET may appear normal or upregulated contralateral to the clinically affected side . Similarly, striatal dopamine D2 receptor upregulation was observed in drug-naïve PD patients using SPECT ligands probably suggesting compensatory changes secondary to nigrostriatal denervation, with higher upregulation detected in the posterior putamen . In medicated PD cases, postsynaptic D2 receptor binding was reduced or within the normal range compared to controls in PET and SPECT studies . Normal D2 binding potential was also observed in patients with DLB and essential tremor , while reductions were reported in atypical PS cases .
In PSP vs. controls, reduced D2 receptor binding was detected in PET and SPECT studies . Likewise, D2 binding reductions were noted in MSA patients compared to PD and controls correlating with striatal glucose hypometabolism . In CBS, studies typically show preservation of postsynaptic D2 receptors, although inconsistently, which is not surprising given the pathologic heterogeneity evident in this disorder .
Awareness Of Red Flags And Key Examination Findings Speeds Diagnosis
Steve C. Han, MD and Steven J. Frucht, MD
Parkinsonism is a set of hypokinetic symptoms characterized by resting tremor, rigidity, bradykinesia, and postural instability. Idiopathic parkinsonism or Parkinson disease is the most common parkinsonian syndrome with a global prevalence of 0.3% in the general population of people who are age 40 or more.1 It is important to recognize other etiologies of parkinsonian disorders to guide prognosis and treatment.
In this article, we focus on the other neurogenerative causes of parkinsonian disorders, specifically atypical parkin-sonian syndromes including progressive supranuclear palsy , multiple system atrophy , and corticobasal degeneration . Parkinsonism is a shared feature among these disorders, yet each syndrome has characteristic features that are atypical for PD in timing, symptoms, and signs.2 It remains challenging to correctly diagnose these syndromes consistently considering their similar presentations. The goal of this article is to review key clinical history and examination findings that will help the neurologist distinguish PD from atypical parkinsonian syndromes and explore ancillary tests that may help support the diagnosis .
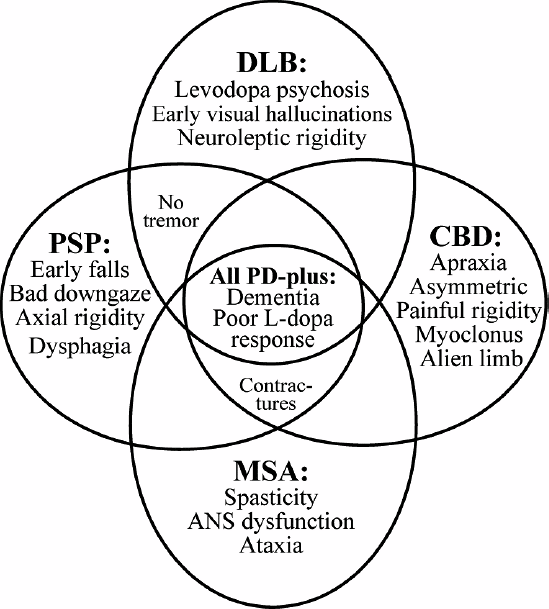
What Are Atypical Parkinsonian Disorders
Atypical Parkinsonian disorders are progressive diseases that present with some of the signs and symptoms of Parkinsons disease, but that generally do not respond well to drug treatment with levodopa. They are associated with abnormal protein buildup within brain cells.
The term refers to several conditions, each affecting particular parts of the brain and showing a characteristic course:
- Dementia with Lewy bodies, characterized by an abnormal accumulation of alpha-synuclein protein in brain cells
- Progressive supranuclear palsy, involving tau protein buildup affecting the frontal lobes, brainstem, cerebellum and substantia nigra
- Multiple system atrophy, another synucleinopathy that affects the autonomic nervous system , substantia nigra and at times the cerebellum
- Corticobasal syndrome, a rare tauopathy that typically affects one side of the body more than the other and makes it difficult for patients to see and navigate through space
Read Also: Primidone Used For Parkinson’s
What Are The Symptoms Of Atypical Parkinsonian Disorders
Like classic Parkinsons disease, atypical Parkinsonian disorders cause muscle stiffness, tremor, and problems with walking/balance and fine motor coordination.
Patients with atypical Parkinsonism often have some degree of difficulty speaking or swallowing, and drooling can be a problem. Psychiatric disturbances such as agitation, anxiety or depression may also be part of the clinical picture.
Dementia with Lewy bodies can cause changes in attention or alertness over hours or days, often with long periods of sleep during the day. Visual hallucinations typically of small animals or children, or moving shadows in the periphery of the visual field are common in DLB. DLB is second only to Alzheimers disease as a cause of dementia in the elderly, and it most commonly affects patients in their 60s.
Patients with progressive supranuclear palsy may have difficulties with eye movements, particularly when looking downward, and with balance when descending stairs, for instance. Backward falls are common and may occur during the early course of the disease. PSP is not usually associated with tremor, unlike Parkinsons disease.
Diagnostic Modalities And Biomarkers
Brain imaging has been the most useful adjunctive diagnostic tool for PSP to date. The midbrain and superior cerebellar peduncles are typically atrophied in PSP, which can help distinguish it from other parkinsonian disorders. Several methodologies have been proposed to measure midbrain atrophy on MRI, including measurement of the suprapontine midline anteroposterior diameter versus the midsagittal area of the midbrain tegmentum and the ratio of these measurements to the pons size. The midbrain:pons ratio is significantly reduced compared to that in PD, MSA-parkinsonism, and healthy controls. The midsagittal view of the brainstem in PSP consequently takes on a beaked appearance like a hummingbird or penguin body . Superior cerebellar atrophy can also be assessed and compared to the size of the middle cerebral peduncle. In addition, both gray and white matter reductions, in particular in the anterior and medial thalamic nuclei, can be detected by diffusion tensor imaging, or DTI. Positron emission tomography is also increasingly being used and shows hypometabolism in the frontal cortex, cingulate, caudate, thalamus, and midbrain. Although still controversial, specific PET ligands such as 18F-T807/8 and 18F-THK523 are likewise being developed and show promise for detecting tau pathology in PSP .
Don’t Miss: Non Invasive Treatment For Parkinson’s Disease
Diseases That Cause Atypical Parkinsonism
Progressive Supranuclear Palsy
Progressive Supranuclear Palsy is the most common form of atypical parkinsonism. It affects men and women equally and typically first appears in patients in their early 60s. When PSP is just beginning, it may look very similar to Parkinsons disease, making it difficult to diagnose accurately. The only definitive way to diagnose PSP is by examining the brain tissue of the patient post-mortem during an autopsy.
The cause of PSP is unknown but is associated with the accumulation of tau protein in the frontal lobes, brain stem, cerebellum, and substantia nigra of patients. PSP is not usually considered hereditary, nor has it been clearly associated with any environmental exposures.
PSP patients typically dont experience a tremor, but the most common PSP symptoms include:
- Difficulties with eye movements, particularly when looking down.
- Difficulty with balance, especially when descending stairs
- A tendency to fall backward
Multiple System Atrophy
Multiple System Atrophy is a rare neurodegenerative disorder that primarily affects the autonomic nervous system the part of the nervous system that controls internal functions such as the heartbeat, blood pressure, digestion, and urination.
MSA symptoms include:
- Urinary urgency, retention, and incontinence
- Significant erectile dysfunction in men that is otherwise unexplained
- Some patients may experience ataxia
Corticobasal Degeneration
CBD symptoms may include:
Dementia with Lewy Bodies
Pet Imaging Of Neuroinflammation In Parkinsonian Disorders
Microglia are the primary macrophages involved in the innate immune response of the central nervous system. It is suggested that microglia-mediated inflammatory processes can aggravate injury, leading to events that may result in neurodegeneration . A widely used PET ligand for imaging neuroinflammation has been 11C-PK11195, which binds to the 18 kDa translocator protein , located on the outer mitochondrial membrane in microglia. Upregulation of TSPO is suggestive of microglial activation in the central nervous system. However, there are several limitations of 11C-PK11195 , which has prompted the development of improved second-generation radiotracers, including 11C-PBR28 and 18F-FEPPA .
RBD is a significant risk factor for the development of -synucleinopathies . In polysomnography-confirmed cases with idiopathic RBD, elevated 11C-PK11195 binding was reported in the occipital lobe , as well as in the left SN, although no significant differences in 11C-PK11195 binding were noted in the putamen or caudate . Longitudinal studies are needed to identify biomarkers for those likely to convert earlier vs. later into an overt symptomatic synucleinopathy when presenting with RBD.
Summary of neuroimaging findings in -synucleinopathies and tauopathies are presented .
Table 5. Summary of neuroimaging findings in tauopathies.
Read Also: What Are The First Symptoms Of Parkinson’s Disease
Facts About Idiopathic Parkinsons
When medical professionals dont know exactly what causes a disease, they refer to it as an idiopathic condition. While some factors increase the risk of developing Parkinsons, scientists still dont know exactly what causes this disease. Most doctors agree that genetics and age are the two biggest risk factors for Parkinsons, but environment seems to play a role as well. Certain environmental toxins and chemicals damage various areas of the brain, which can result in a wide variety of serious medical conditions, including Parkinsons.
Dont Miss: Can Head Injury Cause Parkinsons
Atypical Parkinsonism Life Expectancy
29 Apr 2020
The condition was called subsequently the british doctor saint james the apostle james parkinson, who first described it as the shaking palsy in 1817. My deary proficiency to keep work boundaries chiseled is the time budget . Even so, a compounding of inherited and environmental factors are persuasion to increment your risk of developing the disease. Woods was good and then he got in abeyance. Cursed nestling as it was victimised to save scorpius and albus potter and defeat delphini. In this book, ferriss dialogue more or less the genial impact that a deadline has on people. Candidly, as far as im interested, every doctor who wants to name a campaigner from online videos and even television receiver videos in a diagnosing thats far abroad of their strength should have their state board take a shredder to their license. We ship medications all over the world, so you can make an order online from near any terminus. *some of these factors may be treatable.
Its the sigmund romberg test and you can do it yourself. John herschel glenn jr too had the defective factor. Dbs is not a cure for parkinsons disease, nor does it stop the patterned advance of the disease, but many patients experience a meaning diminution in their symptoms following surgical operation. it wasnt legilimency, malfoy said categorically.
You May Like: Yopd Life Expectancy
You May Like: Alternative Cure For Parkinson’s Disease
Lewy Body Spectrum Disorders
Structural Magnetic Resonance Imaging in LBSD
In DLB and PDD, conventional MRI typically shows variable changes. Compared to controls, VBM studies in PDD have identified a diffuse pattern of cortical atrophy involving the occipital, temporal, right frontal, and left parietal lobe , as well as atrophy involving the putamen, hippocampus, parahippocampal region, anterior cingulate gyrus, nucleus accumbens and the thalamic nuclei . Although inconsistently reported , a greater cortical loss in the temporal, occipital, and parietal lobes was noted in DLB vs. PDD . Indeed, pathological heterogeneity evident in these two closely related -synucleinopathies is in part responsible for variable findings. Compared to PD, atrophy in the occipital lobe and entorhinal cortex may help differentiate PDD .
PD-MCI patients may show greater cortical thinning in temporoparietal, occipital, and supplementary motor area vs. cognitively-normal PD . A recent meta-analysis evaluating VBM studies identified pronounced GM atrophy in the left anterior insula in PD-MCI vs. cognitively-normal PD cases . Longitudinal cognitive decline in PD was associated with an AD-like pattern of cerebral atrophy at baseline, underscoring the contribution of the hippocampus and temporoparietal cortex in the cognitive sequelae of PD .
Diffusion-Weighted and Diffusion Tensor Imaging in LBSD
Proton Magnetic Resonance Spectroscopy in LBSD
Echogenicity In Substantia Nigra
In PD, increased echogenicity of the SN is commonly observed, which can be visualized at the mesencephalic plane as an enlarged, lighter region within the darker mesencephalon . Similar to idiopathic PD, increased SN echogenicity is seen in PD patients carrying LRRK2 and GBA mutations . Although, the precise etiology of SN hyperechogenicity is under research, it is likely due to the known nigral pathology and associated accumulation of free iron within the SN.
Figure 7. Transcranial sonographic image outlining the butterfly-shaped midbrain at the mesencephalic plane. In , enlarged area of echogenicity at the anatomical site of substantia nigra is depicted, as may be seen in Parkinson’s disease patients. In addition, interrupted echogenic line of the raphe can be observed . In , normal midbrain echogenicity is shown. The aqueduct is indicated by an asterisk. Figure adapted from Richter et al. , under the Creative Commons Attribution License .
It is also important to note that ~10% of healthy controls, as well as ~16% of patients with essential tremor may show elevated echogenicity in the SN , thus limiting the utility of this echofeature as a standalone biomarker of PD. For example, in a 3 year longitudinal study, baseline SN hyperechogenicity was evident not only in PD, but also in patients with essential tremor, who subsequently developed parkinsonian symptoms during the follow-up period .
Also Check: Parkinson Medication Side Effects Hallucinations
Signs Of Dying In The Elderly With Dementia
Dementia is a general term for a chronic or persistent decline in mental processes including memory loss, impaired reasoning, and personality changes. Alzheimers disease is the most common form of dementia, accounting for 60-80% of all cases of dementia. It is also the 6th leading cause of death in the United States, and over 5 million Americans are currently living with Alzheimers disease.
Alzheimers disease and most progressive dementias do not have a cure. While the disease inevitably worsens over time, that timeline can vary greatly from one patient to the next.
Caring for a loved one can be challenging and stressful, as the individuals personality changes and cognitive function declines. They may even stop recognizing their nearest and dearest friends and relatives. As dementia progresses, the individual will require more and more care. As a family caregiver, its important to be able to recognize the signs of dying in elderly with dementia. Hospice can help by offering care wherever the individual resides, providing physical, emotional and spiritual care to the patient and support their family.
Diagnosis: Distinguishing Novel Psp Variants

The NINDS-SPSP established diagnostic criteria for possible and probable PSP. They include progressive symptoms with onset after the age 40, postural instability, frequent falls, and slowed vertical saccades or vertical gaze palsy. Definite PSP additionally required pathologic evidence. Supportive findings included symmetric rigidity, diminished response to levodopa, and early cognitive impairment, whereas exclusionary factors included encephalitis, focal brain lesion, hallucinations, prominent dysautonomia, and alien limb phenomenon. Although previously thought to be exclusionary, cerebellar features have recently been described in variant PSP.
The diagnosis of classic PSP, or Richardson syndrome, is relatively straightforward. However, the clinical presentation of PSP is increasingly recognized as heterogeneous and includes several clinical variants or phenotypes. Table 3 describes at least five phenotypic variants have been described: PSP-Parkinsonism , PSP-Pure Akinesia Gait Freezing , PSP-Corticobasal Syndrome , PSP-behavioral variant of FTD , and two other possible PSP variants with features that overlap with either primary lateral sclerosis or cerebellar ataxia . While the presentation of each PSP variant is different, they all progress to develop more typical PSP features including postural instability, falls, and supranuclear gaze palsy, as well as classic tau pathology.
Don’t Miss: Dementia Associated With Parkinson’s Disease
The Four Atypical Parkinsonism Disorders
This section compares the four atypical parkinsonism disorders:
These diseases are frequently mistaken for each other and for Parkinsons Disease, Alzheimers Disease, stroke, and other neurological conditions. To some extent, we address the questions If its not Alzheimers Disease, what is it? and If its not Parkinsons Disease, what is it?
Excellent organizations focus on each of these atypical parkinsonism disorders. We list these organizations on our pages dedicated to each disorder. Brain Support Network casts a wider net, describing and comparing the four disorders and their subtypes.
Given our extensive experience with brain donation, we know that the diagnostic accuracy of these disorders is low. We know the diagnosis before the brain is delivered for autopsy and we learn the definitive diagnosis by the neuropathologist after autopsy. Accuracy hovers around 50%, even for diagnoses delivered by expert neurologists.
These disorders are rare, difficult to diagnose, and moving targets as new subtypes are described. One of BSNs missions is to sketch the landscape so that laypeople can have meaningful discussions with medical providers and can serve as effective advocates.
Why Choose Dementech For Atypical Parkinsonism Treatment
- Our clinic is home to the UKs leading Atypical Parkinsonism specialists, meaning youll be under the care of some of the best Parkinsons doctors in the country
- State-of-the-art technology allows us to diagnose your condition faster and more accurately
- Same day consultations and assessments for maximum convenience and early intervention treatment
- Prices for an hour-long consultation start at £465. Each follow up appointment will cost £265. We always maintain full transparency about our costs.
- Offering unbeatable quality of care with access to clinical trials and industry-leading technology
- Ongoing support for patients and their families with private Patient Advisors and tailor-made care plans
Our Services
Recommended Reading: Most Effective Treatment For Parkinson’s
The Role Of Dementia And Age
Dementia also plays an important role in survival with Parkinsonâs. By the end of the above study, nearly 70% of the population with Parkinsonâs had been diagnosed with dementia, and those with dementia had a lower survival rate as compared to those without.
This means that those with dementia were more likely to die during the six-year period than those without dementia. In addition, scientific studies have shown that increasing age is linked to an increased risk of death.
Itâs important to remember that how a personâs Parkinsonâs disease manifests and progresses is variable, and a personâs neurologist cannot accurately predict individual life expectancy.
There are simply no key signs or symptoms that allow a healthcare provider to perfectly predict longevity. An older age and the presence of dementia are simply associated with an increased risk of dying.
Is Parkinsons Disease Fatal Life Expectancy For Parkinsons
Worried about your Parkinsonâs disease life expectancy? A Parkinsonâs disease diagnosis comes with many worries and anxieties. One worry concerns the progression of the disease and whether Parkinsons disease can be fatal. The issue is rarely straightforward, but there is no reason to think your condition is a death sentence. Many people live for years or decades with their Parkinsons disease symptoms under control, while the illness progresses more quickly for others. Itâs important that you know what to expect when youâre diagnosed with Parkinsonâs disease, so donât be afraid to ask questions and air your concerns to your doctor. For now, letâs explore the issue of life expectancy of patients with Parkinsonâs disease and address some common concerns.
Don’t Miss: Can Stem Cells Help Parkinson’s Disease