What Are Stem Cell Lines
A stem cell line is a family of constantly dividing cells, the product of a single parent group of stem cells. They are obtained from human or animal tissues and have been manipulated in a laboratory so that they have the ability to divide almost indefinitely, creating the line.
Because stem cell lines produce so many copies of themselves , this means that scientists have a large bank of cells for their research and are less likely to need to take cells from an embryo repeatedly. Of course, it is important that researchers are also able to stop such cell lines dividing at some point so that they can generate tissue specific cells, such as for the brain in the case of Parkinsons treatment. The challenge for researchers is to discover how they can control the process which causes stem cells to differentiate.
Parkinsons Disease: How Could Stem Cells Help
What do we know?
Tremors, muscle rigidity and other symptoms of Parkinsons disease are caused by the death of dopamine-producing neurons in the brain. Dopamine producing neurons throughout the brain are affected, but the substantia nigra is the primary brain region where neurons are lost.
People affected by PD often develop abnormal protein clumps in their brain called Lewy bodies. These clumps are made of a protein called alpha-synuclein.
Levodopa is the primary drug used to treat PD. Levodopa is converted into dopamine when in the body, which compensates for lost dopamine-producing neurons.
What are researchers investigating?
Approximately 5% of people with PD have inheritable gene mutations linked to PD. Researchers are investigating what causes PD in the other 95% of patients in clinical studies, animal models and cell models.
Transplantation of young brain cells from human foetuses into people with PD has shown promising results in previous clinical trials. The current TRANSEURO study is re-examining this treatment method with the aim of minimising side effects and measuring efficacy.
Scientists can now make dopamine-producing neurons from both human embryonic stem cells and human induced pluripotent stem cells . Neurons made from human ESCs and iPSCs mature into human dopamine-producing neurons, survive and function after transplantation into mouse, rat and monkey models of PD.
What are the challenges?
Replacing lost cells
Pure Substantia Nigra Mda Neuron
Recently, single cell gene profiling has been used to define subtype compositions during mouse and human midbrain development . Such technology extended the previous molecular definition of mDA neurons and enabled to further divide SN and VTA region into seven distinct molecular clusters . However, to what extent these molecular findings can be translated into hPSC-derived mDA neuron development remains unexplored. This is illustrated when profiling of hPSC-derived mDA neurons in La Manno et al., which seem to recapitulate key stages of in vivo ventral midbrain development. However, those cell preparations expressed many poorly defined radial glial and neuroblast markers and differed from the in vivo phenotypes in gene expression . Additionally, ALDH1A1 was not expressed in any of those PSC-derived mDA cells. This result may be due to the in vitro culture environment that does not fully support mDA neuron development or lack of proper induction of certain subtype-specific genes. In either case, those results indicate that there is considerable room for further improvements in mDA neuron derivation and maturation strategies.
Don’t Miss: Vitamin E And Parkinson’s Disease
How Do Mesenchymal Stem Cells Work In The Body
Mesenchymal stem cells utilize their self-renewal, immunomodulatory, anti-inflammatory, signaling, and differentiation properties to influence positive change within the body. Mesenchymal stem cells also have the capacity to self-renew by dividing and developing into multiple specialized cell types present in a specific tissue or organ. Mesenchymal stem cells are adult stem cells, meaning they present no ethical concerns, MSCs are not sourced from embryonic material.
Maturity Of Transplantable Cells
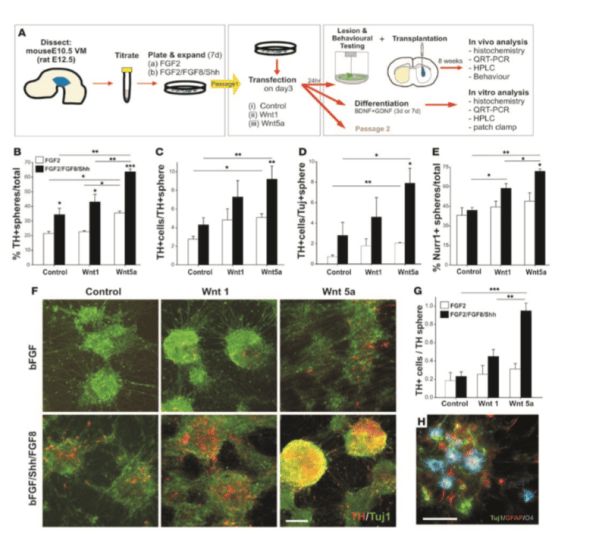
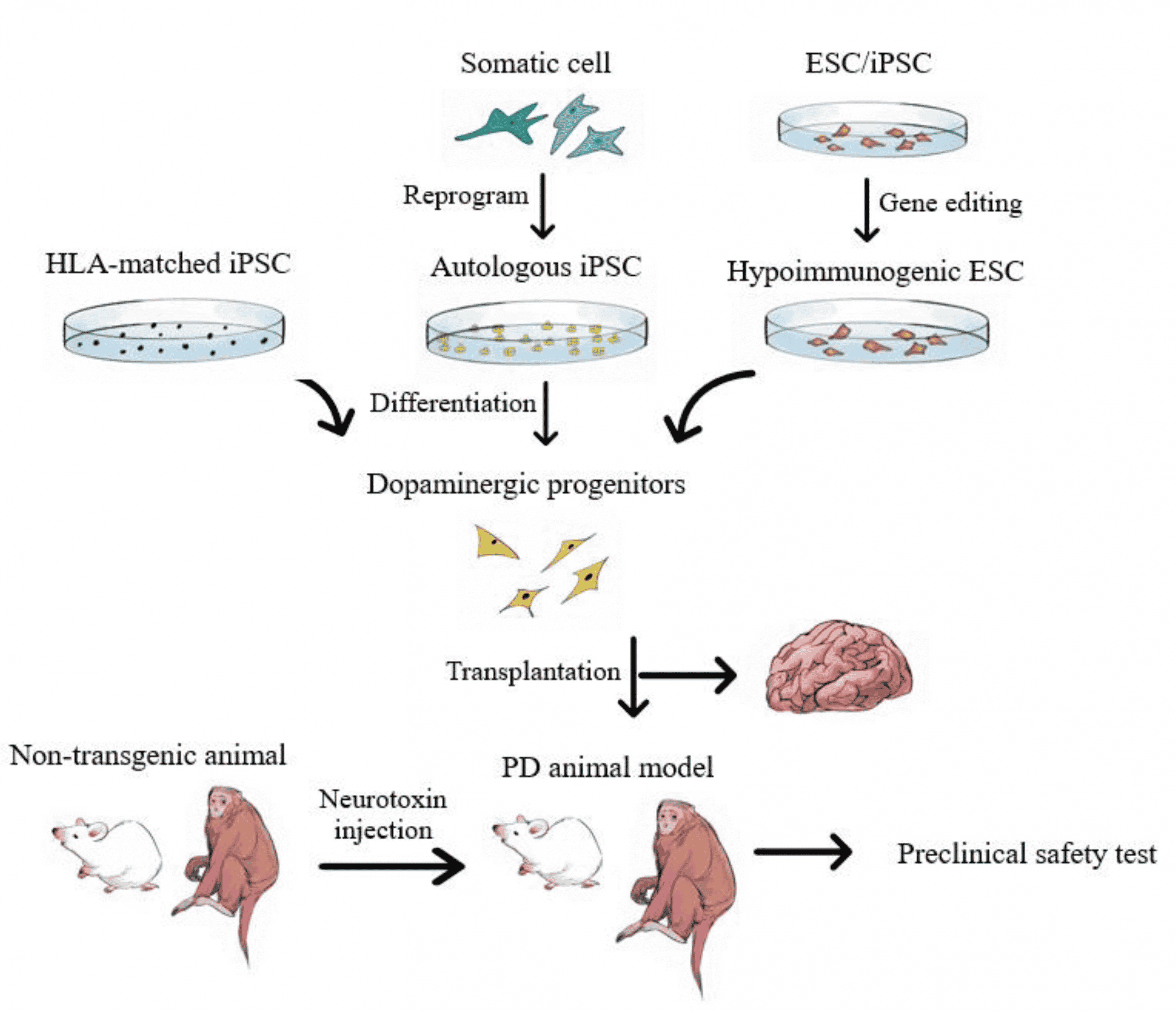
Figure 6. Schematic describing the process for autologous cell therapy for Parkinsons disease. Blood is collected from patients by venipuncture. Peripheral blood mononuclear cells are isolated and the specific somatic cell population for reprogramming is expanded. Reprogramming factors are introduced and clonal lines expanded for quality control testing. Clones that pass all quality control steps are used for mDA differentiations. The mDA neurons are cryopreserved and the batches quality control tested prior to autologous transplantation.
Don’t Miss: Do Women Get Parkinson’s
Direct Conversion Of Astrocytes
Another approach that would also eliminate the need of immune suppression is the direct conversion of somatic cells to DA neurons in vivo using virus technology. The current approaches for PD aim to convert astrocytes to DA neurons . This could be a potentially interesting approach but is still in early exploratory stages. A potential pitfall of this strategy is the local loss of the astrocytes that are reprogrammed to neurons and the potential associated problems with this local astrocyte loss in a human brain. Astrocytes have numerous important functions, and many of these functions are essential for brain homeostasis and neuronal health. For example, they provide neurotrophic and metabolic support, regulate synaptogenesis and synaptic function, contribute to the blood-brain-barrier and play an important role in limiting the spread of local immune response initiated my microglia, preventing cell damage to surrounding tissue. There is also a cellular and molecular diversity among astrocytes, thus understanding what cells and functions are lost would be important to predict how a conversion of local astrocytes to DA neurons might affect the function of the brain in a PD patient .
Where Do Stem Cells Come From
Various types of stem cells are found at different stages of human development and in different parts of the body, all of which are of interest to researchers.
Embryonic stem cells: these stem cells are found in fertilised eggs that are only a few days old. These are the only cells that can develop into any type of cell within the body. The abundance of stem cells decreases as the embryo grows and stem cells become specialised cell types that form parts of our body.
Whilst embryonic stem cells are very promising as a treatment for Parkinsons, they also have the risk of uncontrolled growth in the body which could lead to the formation of tumours. Much more research is needed in order for scientists to understand how stem cells work and how they may be used to produce treatments for Parkinsons and many other medical conditions.
Adult stem cells: although adult stem cells exist, they are found in quite small numbers in certain parts of the body and do not multiply quickly. Adult stem cells have been used to treat some conditions for example bone marrow transplants to treat leukaemia. However, it is not currently possible to change adult stem cells into nerve cells suitable as a treatment for Parkinsons.
Bone marrow: these stem cells are found in our bone marrow and bones.
Also Check: Parkinson’s Off Time Symptoms
Studies Show Promising Results
“Considering the ability of MSCs to secrete neurotrophic factors, modulate inflammation, and possibly even act as mitochondria âdonorâ, it comes as no surprise that there is a lot of interest in the use of MSCs in the treatment of Parkinsons Disease, and a multitude of animal studies has shown promise. Treatments have resulted in improvement of motor function, protection of the nigrostriatal system, and improved striatal dopamine release in several studies using toxic lesion rodent models of Parkinsons Disease. Similar effects were reported with umbilical cord-derived MSCs with or without prior differentiation. For example, a recent study reported improvement of motor function, reduced microglial activation, and decreased loss of TH immunoreactivity, associated with local production of trophic factors.
Learn more about DVC Stem’s protocol for Parkinson’s Disease here:
References:
Venkataramana, N. K., Kumar, S. K. V., Balaraju, S., Radhakrishnan, R. C., Bansal, A., Dixit, A., ⦠Totey, S. M. . Open-labeled study of unilateral autologous bone-marrow-derived mesenchymal stem cell transplantation in Parkinson’s disease. Retrieved from https://www.sciencedirect.com/science/article/pii/S1931524409002205#!
Unified Parkinson’s Disease Rating Scale. . Retrieved from https://www.sciencedirect.com/topics/medicine-and-dentistry/unified-parkinsons-disease-rating-scale
About the author
Summits Role In The Development Of The Autologous Ipsc
- Summit was founded in 2011 as a grass roots volunteer organization comprised of patients, medical and scientific professionals and community members with a desire to raise funds for a stem cell-based solution to Parkinsons disease.
- Summit paired with Drs Jeanne Loring and Andres Bratt-Leal, then located at the Scripps Research Institute in La Jolla, CA to develop this stem cell based therapy.
- Over the years, Summit raised millions of dollars to support this project.
- As the project progressed, it became clear that in order for the project to be successful, a private sector biotech company would have to be built in order to raise the type of funds that would be necessary to bring the project from bench to bedside.
- In 2018, Aspen Neuroscience was created to bring the autologous iPSC-derived dopamine neuron replacement therapy to the Parkinsons patient population and now the project is on an accelerated path toward FDA-approved clinical trials.
- Summit continues with passionate dedication to advocate for persons living with Parkinsons and other neurodegenerative diseases.
- We update patients about the autologous iPSC-derived dopamine neuron replacement therapy, provide financial assistance to patients who want to participate in the trial and fund evidence based regenerative medical therapies for Parkinsons and other neurodegenerative diseases.
Also Check: Parkinson’s Loss Of Taste
Explored Cells For Crt
Owing to recent biological breakthroughs associated with advanced technologies, new cell sources, such as stem cells, have emerged . It is important to bear in mind that the concept of the ‘perfect’ cell source for DA neuron generation might be a utopic concept â that is, each type of stem cell possesses specific advantages and disadvantages. Unique methodologies have been developed in order to coax specific differentiation processes into DA neurons, including genetic manipulation, exposure to a variety of morphogenetic factors or chemical compounds. To date, a host of different cells has been explored in the search for new DA neuron sources. Hereafter, we will review the experiences gained through the years with some of these cells, towards an efficient and secure DA neuron generation.
How Might Stem Cell Therapy Benefit Parkinsons
Researchers are exploring various approaches to use stem cells to treat Parkinsons disease.
The current idea is to introduce stem cells directly into the affected areas of your brain where they can transform into brain cells. These new brain cells could then help regulate dopamine levels, which should improve the symptoms of the disease.
Its important to note that experts believe this would only be a treatment for Parkinsons disease and not a cure.
While stem cell therapy has the potential to replace the brain cells destroyed by Parkinsons disease, the disease would still be present. Parkinsons disease would likely destroy the implanted stem cells eventually.
Its unclear right now whether stem cell therapy could be used multiple times to continue to reduce symptoms of Parkinsons disease or if the effect would be the same after multiple procedures.
Recommended Reading: Drugs For Parkinson’s Psychosis
A Patient Disease Model
HSCI Principal Faculty member and Director of the Neuroregeneration Research Institute at McLean Hospital Ole Isacson, MD, with funding from HSCI, the Harvard Miller Consortium for the Development of Nervous System Therapies, and the National Institutes of Health, has generated brain cells that produce dopamine, collected from the skin cells of patients with Parkinsons.
Isacson orchestrated the transformation by biologically reprogramming the mature skin cells into induced pluripotent stem cells, and then encouraging the stem cells to become dopaminergic neurons. Neurons were also made from skin cells collected from individuals with genetic mutations associated with high risk for Parkinsons disease.
The creation of this in vitro disease model provides a powerful platform for studying Parkinsons outside of the body. In the journal Science Translational Medicine, Isacson and his team reported that many of the mutations implicated in Parkinsons affect the function of the mitochondria, the cellular organelle responsible for energy production. In collaboration with HSCI Director of Translational Medicine Lee Rubin, PhD, Isacsons lab started to identify compounds that could eliminate disease symptoms in cell lines derived from people carrying Parkinsons mutations.
New Cell Surface Markers
- American Society for Biochemistry and Molecular Biology
- Summary:
- Researchers describe a new set of cell surface markers on dopaminergic progenitor cells, which allow isolation of a more beneficial population of induced neurons for cell replacement therapy. Animals that received transplanted cells that had been selected for the new marker fared better than their counterparts with a typical transplant.
Parkinson’s disease is a neurodegenerative disease that affects dopamine signaling neurons in patients’ brains. Cell-replacement therapy shows some promise as a treatment for Parkinson’s. A recent paper in the journal Molecular & Cellular Proteomics reports a technical advance in selecting cells to use in this therapy.
Cell-replacement therapy involves differentiating stem cells into dopamine-signaling, or dopaminergic, neurons and transplanting them into a patient’s brain to replace dying neurons. However, the variability of differentiated cells — including contamination with other neuronal cell types or residual undifferentiated stem cells — can affect transplantation outcomes. In clinical trials in the 1990s, for example, such contamination gave some patients severe dyskinesia, uncontrollable jerky movements that were worse than the movement problems caused by Parkinson’s disease.
The team’s isolation procedure uses may be an important step toward more successful cell-replacement therapy.
Story Source:
Read Also: How Long Has Michael J Fox Had Parkinson
What Is Stem Cell Therapy
Stem cells are special because theyre undifferentiated, meaning they have the potential to become many types of specialized cells.
You might think of stem cells as natural resources for your body. When your body needs a specific type of cell from bone cells to brain cells an undifferentiated stem cell can transform to fit the need.
There are three main types of stem cells:
- Embryonic stem cells: These cells are pluripotent, meaning they can transform into the many types of cells found in your body. As the name suggests, theyre found in embryos.
- Somatic stem cells: Also called adult stem cells, these mostly perform repair functions. They can still transform, but not into as many types of specialized cells as embryonic stem cells can.
- Induced pluripotent stem cells : These stem cells are made by genetically changing cells that have already matured.
Stem cell therapy is the use of stem cells usually from a donor, but sometimes from your own body to treat a disorder.
Because Parkinsons disease leads to the death of brain cells, researchers are trying to use stem cells to replace brain cells in the affected areas. This could help treat the symptoms of Parkinsons disease.
Next Step: Human Trials
Dr. Kordower told MNT that the results of this study give him great confidence going forward into patients.
Dr. Kordower will be a principal investigator in a clinical trial that he expects to take place in 2023, which will study a specific population of individuals with PD who have mutations in the Parkin gene .
These individuals experience degeneration of the dopamine system. While they experience motor dysfunction typical with PD, they do not develop cognitive decline or dementia. So, that makes the perfect test to see whether cell replacement strategies can be helpful, Dr. Kordower told MNT.
If the trial is successful, larger trials may follow in a broader population of people with PD. However, it is important to note that while the findings from this study are promising, results from animal models do not always translate into human clinical trials.
Recommended Reading: Is Acting Out Dreams A Sign Of Parkinson’s
The Next Generation Of Trials
Studer was part of the initial studies involving fetal tissue in the 1980s and 1990s, and knew from the start that the work was more of a proof of principle than a solution for people with Parkinsons. For me it was clear that a fetal transplant isnt a long-term solution because of ethical, legal and practical issues. Because this procedure requires 4 to 12 fetuses per patient, there was no way they could treat thousands, let alone tens of thousands, of people that way. Instead, Studer turned to stem cells.
Immunosuppression is a particularly important element of BlueRocks approach, because it relies on a single cell line that cannot be adjusted to more closely resemble the recipients own tissues. A group led by stem-cell scientist and neurosurgeon Jun Takahashi at Kyoto University in Japan is attempting to provoke a lesser immune response by pairing transplant recipients with cells that are less likely to be rejected. The researchers are using cell-surface proteins, called major histocompatibility complexes , that are recognized by the adaptive immune system and can have varying levels of compatibility from one person to another. Rather than using frozen cell lines, Takahashi and his colleagues are creating a fresh batch of MHC-matched cells for each transplant.
When Will Stem Cell Therapy Be Available As A Parkinson’s Treatment
Whilst there has been considerable progress in stem cell research in the last decade, particularly for the treatment of blood and immune system disorders, scientists are still some way from being able to start clinical trials using stem cell therapy for Parkinsons. No-one can predict how long it is likely to take for stem cell therapy to be a viable treatment for Parkinsons.
Also, even if a therapy is approved, it is unlikely to work for everyone just as no one medication is suitable for everyone.
At this stage, scientists do not know which type of stem cell, if any, may eventually lead to a successful treatment or cure.
The key challenges for scientists at present are:
- to understand the way cells grow and differentiate
- to identify methods to differentiate the stem cells into the cell types needed in the brains of people with Parkinsons
- to establish the best ways of getting stems cells into the right part of the brain.
Also Check: How To Be Tested For Parkinson’s