Altered Gut Metabolites In Pd
Intestinal microorganisms interact with the host through secreted toxins, by-products, and metabolites to modulate immune responses, endocrine secretion, metabolism, and neurotransmission . Analyses of predicted functional pathways of the gut microbiome have shown that metabolism involving lipopolysaccharide , carbohydrates, amino acids, lipids, energy, cofactors, and vitamins is altered in PD . Consistently, gut microbial metabolites have been observed to be different between PD patients and controls in different biofluids or samples, including feces, plasma, and cerebral spinal fluid . Some gut metabolites could discriminate PD patients from unaffected controls, whereas some correlate with disease severity and progression, suggesting their participation in the disease mechanism and the potential to serve as disease biomarkers.
Table 1 Alteration of microbial metabolites in Parkinsons disease
Altered microbial metabolism may reflect diverse interactions between the heterogenous host genetic background and gut environmental factors contributing to disease formation in individual patients. Notably, altered metabolomes are not unique findings in PD, but are also observed in other neurodegenerative diseases, including Alzheimers disease and atypical parkinsonism, with some similar and different results indicating common and distinct disease processes of neurodegeneration .
Role Of The Ldr In The Development Of Motor Fluctuations
Fig. 1
Mean peak and baseline tapping speeds to the levodopa infusion on day 1 and day 4 and over 4 years of therapy with levodopa . The difference between peak and baseline tapping speeds is the magnitude of the SDR, which progressively increases to the levodopa infusion on day 4. Reproduced from Nutt et al. .
Similar results were reported by Zappia et al. who found that the duration of the SDR did not significantly change within the first year of therapy, but that 24% of patients lost the LDR to levodopa. These studies demonstrate the pivotal roles of the LDR and the magnitude rather than duration of the SDR in the development of motor fluctuations in the early years of levodopa therapy. They further suggest that when a sustained LDR is present, the SDR is usually masked and patients may therefore be classified clinically as stable responders to levodopa therapy even though they are experiencing fluctuations. As the LDR is progressively lost, patients lose the smooth drug effect and the magnitude of the SDR increases . Patients are then clinically observed to become fluctuators because the degree of benefit is now dependent on the magnitude of the SDR. Overall, the available evidence suggests that in the earlier stages of PD, where the difference between the ON and OFF states is less pronounced, any fluctuations in levodopa response are not noticed by the patient and therefore not reported.
Fig. 2
Clinical Relevance Of Early Recognition Of Wearing
In clinical practice, treatment is initiated once the compensatory mechanisms operative in early stages of the loss of the nigrostriatal pathway have failed. As a result, there is already a reduced capacity at the presynaptic dopamine terminal level to compensate for changes in dopamine availability associated with fluctuations in plasma and brain levodopa levels after oral administration. Therefore, the output of basal ganglia motor oscillates precariously between various abnormal states. The critical and therapeutically relevant point is that standard short-acting levodopa formulations lead to levels of striatal dopamine and dopamine receptor stimulation different from those prevailing under normal conditions and oscillating between subphysiological and supraphysiological levels. This being a reflection of the peaks and troughs associated with changes in plasma levodopa concentrations that characterize the use of standard levodopa preparations. Thus, standard levodopa administration does not restore the normal physiology of the basal ganglia , but induces, through pulsatile stimulation, molecular abnormalities such as phosphorylation of NMDA subunits and upregulation of AMPA receptors in medium spiny striatal neurons that underlie wearing-off .
Don’t Miss: Natural Foods For Parkinson’s Disease
Altered Gut Microbiota In Pd
Notably, emerging evidence indicates that protein nucleation and aggregation may be influenced by an extracellular amyloid protein called curli, which is secreted by Escherichia coli, inducing neuronal deposition of alpha-synuclein in the gut and promoting neurodegeneration . The abundance of E. coli at the colonic mucosa correlates with enteric alpha-synuclein deposition in PD patients . Curli is an amyloid protein that bacteria secrete for surface adherence and biofilm formation . Induction of abnormal alpha-synuclein aggregation by curli has been demonstrated in vitro, and ingestion of curli and curli-producing bacteria accelerates enteric and central alpha-synucleinopathy, as well as neuroinflammation, dopaminergic neuronal degeneration, and motor dysfunction, in transgenic rodent models of PD . Therefore, distinct gut microbiota species result in a pro-inflammatory status in the intestinal tract or promote enteric alpha-synuclein aggregation to facilitate the occurrence and progression of PD.
Postsynaptic Mechanisms In Wearing
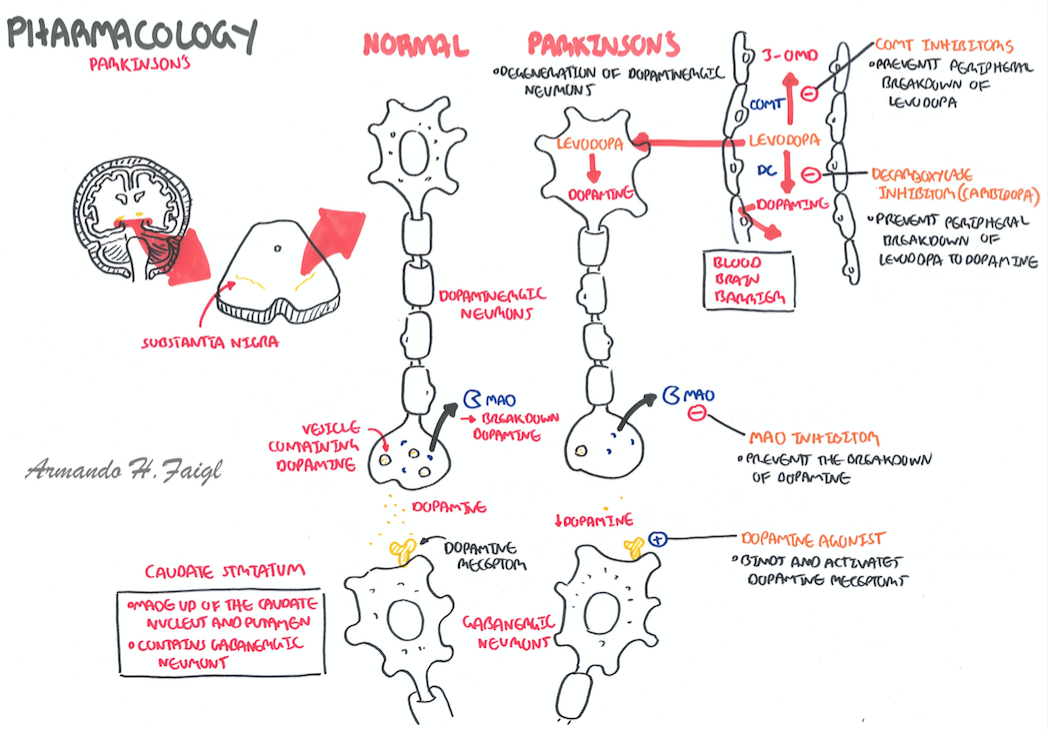
To evaluate the relevance of postsynaptic mechanisms, it is necessary to break down the wearing-off into its LDR and SDR components. Strong support for the involvement of postsynaptic mechanisms comes from the slow decay of the LDR on withdrawing dopamine agonist treatment in patients with de novo PD. For example, the time taken for motor symptoms to deteriorate back to baseline after stopping treatment with ropinirole was 6.2 ± 1.7 days and 9.0 ± 1.9 days with the short-acting agonist lisuride . Interestingly, similar studies in de novo PD patients with the very long-acting dopamine agonist cabergoline showed a shorter LDR compared to short-acting lisuride. From these results, it can be concluded that dopamine agonists have LDR effects that are similar to levodopa and that postsynaptic effects must contribute. We suggest that these postsynaptic changes include complex alterations in genes and protein at the striatal level mediating receptor and intracellular activity and also functional abnormalities in basal ganglia output pathways .
Don’t Miss: Do You Have Pain With Parkinson’s Disease
Can We Put The Brakes On Parkinsons Progression
Researchers are lasered in on slowing and someday stopping Parkinson’s disease in its tracks. Explore what they’ve discovered, see what the future might hold and learn how some of the strongest weapons in the fight against Parkinson’s progression are practices you can put in place today.
This article is based on Can We Put the Brakes on PD Progression, a Parkinsons Foundation Expert Briefing webinar presented by Joash Lazarus, MD, Multiple Sclerosis Center of Atlanta.
PD symptoms stem from a protein, called alpha-synuclein, that clumps and accumulates in certain areas of the brain. This process depletes dopamine, which is critical to many body processes, including smooth, coordinated movements. Though dopamine declines for everyone who lives with Parkinson’s, each person experiences disease symptoms differently.
Parkinson’s symptoms can impact your life in numerous ways. Using a range of therapies and supports as needed can make all the difference. Personalized medicines, social support groups, mental health care and participation in clinical trials have all shown benefit to people with Parkinson’s.
But is there a way to slow Parkinson’s progression? While scientists are evaluating everything from medications to mindfulness practice for clues, they’ve discovered some of the biggest benefits start at home.
Healthy Eating and Regular Exercise: A Powerful Combo
Here’s how to make exercise work for you:
Exploring Therapy Advances
Rasagiline
Levodopa
Deep Brain Stimulation
Want To Learn More About The Latest Research In Parkinsons Disease Ask Your Questions In Our Research Forum
Therefore, whether levodopa has an effect on the progression of Parkinsons disease beyond its immediate benefit with respect to symptoms remains unknown, scientists wrote.
Now, researchers from the University of Amsterdam designed a multicenter, randomized, placebo-controlled, delayed-start trial to assess levodopas effect on patients with early Parkinsons disease who had insufficient disability to receive anti-Parkinson medication: the Levodopa in Early Parkinsons Disease study .
Patients who had received their diagnosis within the previous two years were randomly assigned to an early-start group : levodopa in combination with carbidopa for 80 weeks or to a delayed-start group : placebo for 40 weeks followed by levodopa in combination with carbidopa for 40 weeks.
During Phase 1 , patients received levodopa or placebo. During Phase 2 patients in both trial groups received levodopa. Assessments were made at baseline and at weeks 4, 22, 40, 44, 56, 68, and 80.
The studys primary endpoint was the difference in the mean change, from trial initiation to week 80, in the total score on the Unified Parkinsons Disease Rating Scale . That tool assesses both motor and non-motor symptoms associated with Parkinsons disease .
Secondary outcomes included the progression of symptoms between weeks 4 and 40 and between weeks 44 and 80, as measured by the category scores on the UPDRS disability cognitive impairment depression and disease-related quality of life.
Don’t Miss: Drugs For Tremors Of Parkinson’s
No Effect Of Levodopa On Parkinson’s Progression
Use of levodopa in early Parkinson’s disease does not have any disease-modifying effect, either beneficial or detrimental, a new study suggests.
“There was no difference in Parkinson’s symptoms or levodopa side effects at 80 weeks in those patients who started taking the drug at week 1 and those who started at week 40,” senior author Rob de Bie, MD, PhD, University of Amsterdam, the Netherlands, told Medscape Medical News.
“This suggests that levodopa does not have any effect on disease progression either positive or negative.”
He explained that patients and physicians can be reluctant to start levodopa because there is a fear that prolonged use may be associated with faster disease progression, a wearing off of efficacy, or increased side effects.
“But our data give us reassurance that levodopa does not increase disease progression,” he said.
“This should have a big impact on clinical practice,” he added. “Patients can now start taking levodopa with confidence whenever they need to control symptoms without the worry that it may be having an adverse effect. The disease will still progress, and the levodopa may need to be used more frequently, but this does not appear to be related to past use of the drug.”
The study was January 24 in the New England Journal of Medicine.
In an accompanying editorial, Susan Bressman, MD, and Rachel Saunders-Pullman, MD, MPH, Icahn School of Medicine at Mount Sinai, New York City, say this trial supports current practice.
No Effect On Progression
In this new trial, after 40 weeks the researchers found that those on levodopa had improved symptoms compared with those on the placebo. But at the end of the trial there were no significant differences between the 2 groups and those who had taken levodopa for 80 weeks did not experience more side effects.
Dr Katherine Fletcher, our Research Communications Officer, says:
“Levodopa is an important tool for managing Parkinson’s symptoms. But, there are outstanding research questions about how levodopa impacts the progression of Parkinson’s and when people should start the treatment.
“This new research suggests that levodopa does not alter the progression of the condition within the tested timescale. More research into different doses and timescales would be needed to assess this further.
“If people are concerned about their Parkinson’s medication please seek healthcare advice.”
Don’t Miss: Canes For Parkinson’s Patients
Levodopa For Early Parkinson Disease: Some Answers More Questions
Does early initiation of levodopa delay disease progression? Dutch researchers sought to confirm a neuroprotective effect suggested by a previous trial.
RESEARCH UPDATE
A new study suggests that early use of levodopa for treating Parkinson disease has neither disease-modifying properties, nor is it detrimental to disease course. The results answer some questions but raise others about the role of levodopa in PD.
The study was recently published in the New England Journal of Medicine.1
We conclude that treatment with levodopa at a dose of 100 mg three times per day in combination with carbidopa at a dose of 25 mg three times per day had no disease-modifying effect, either beneficial or detrimental, on early Parkinsons disease among patients who were evaluated over the course of 80 weeks, wrote first author Constant V. M. Verschuur, MD, of the University of Amsterdam, and colleagues with the Levodopa in Early Parkinsons Disease Study Group.
Levodopa is usually reserved for treatment of motor symptoms later in the course of PD. Experts have long assumed that the drug only treats the symptoms and does not actually improve the underlying disease process.
However, results from the Earlier vs Later L-DOPA study suggested that levodopa may also have disease-modifying properties. Somewhat confusingly, results from that trial suggested that levodopa may also have detrimental effects on the underlying pathophysiology of PD.2
Results
Take-home points
What Does Current Guidance Say On This Issue
The NICE guideline recommends levodopa as a first-line treatment in the early stages of Parkinsons to control problems with movement if symptoms are affecting the quality of life. It does not discuss delayed treatment with levodopa.
If motor symptoms are not affecting the quality of life, the guideline recommends considering other drugs such as dopamine or monoamine oxidase inhibitors based on individual circumstances and preferences.
Recommended Reading: Fatigue In Parkinson’s Disease
Does Levodopa Slow The Progression Of Parkinsons Disease
Levodopa does not slow or reduce the progression of Parkinsons disease. In a clinical trial, levodopa + carbidopa was found to have no disease-modifying effect when it was used in patients with early Parkinsons disease compared with patients who started it later on in the course of their disease.
- Food and Drug Administration . Sinemet. Available from: . .
- US National Library of Medicine. MedlinePlus. Levodopa and Carbidopa. Available from: . .
- American Parkinson Disease Association. Carbidopa/Levodopa: Answers To Frequently Asked Questions. May 21, 2019. Available from: . .
- European Parkinsons Disease Association . Motor symptoms. Rigidity. Available from: . .
- European Parkinsons Disease Association . Motor symptoms. Bradykinesia. Available from: . .
- Verschuur CVM, Suwijn SR, Boel JA, et al. Randomized Delayed-Start Trial of Levodopa in Parkinson’s Disease. N Engl J Med. 2019 380:315-324. doi:10.1056/NEJMoa1809983.
Role Of Pulsatile Dopaminergic Stimulation In Motor Fluctuations
The role of the nigrostriatal dopaminergic system is primarily to exert a modulatory effect on basal ganglia circuitry, particularly the striatum, but also on other nuclei of the basal ganglia and on thalamic and brain stem regions . Phasic release of dopamine occurs in response to the firing of dopaminergic neurons, and this is mediated by spike-dependent release of dopamine into the synaptic cleft resulting in potent but brief activation of postsynaptic dopamine receptors . Under normal circumstances, neuronal activity adapts quickly to phasic release and firing stops when events lose novelty or relevance . However, the loss of phasic dopamine release is important and it is likely to be responsible for some specific motor and non-motor problems in PD, such as learning deficits, anodynia and, more relevantly in the context of this review, levodopa-induced motor complications.
Fig. 3
You May Like: Does Parkinson’s Always Get Worse
What Is The Clinical Relevance Of These Findings
Stratification, or defining different subcategories, is key to better understanding disease mechanisms and kinetics in PD, predicting disease course and ultimately delivering personalised management strategies. The emerging focus of PD trial design is on early motor disease, including novel immunomodulatory therapies that require intensive and invasive monitoring. Traditionally, little account has been taken of disease heterogeneity in early PD when selecting patients for randomised, placebo-controlled studies. However, our results show that baseline phenotype is associated with variable rates of subsequent motor progression, although confounded by potential medication response effects. The mean difference in UPDRS motor scores between the fastest and slowest motor progression subtypes in Tracking Parkinsons was 2.6 points, equivalent to the primary hierarchical endpoint of several studies, including the ADAGIO study. Recruitment without taking into account heterogeneity and potential sources of recruitment bias may lead to less efficient designs, though there are various trade-offs between the cost of selecting patient subgroups, the sample size required for demonstrating a reduction in disease progression and increasing the length of follow-up.
Pathophysiological Mechanisms In Wearing
A wearing-off effect is the normal and predictable response of any sensitive pharmacological system when activated with a potent but short-acting agent. There are numerous and well-accepted examples in general pharmacology and clinical practice. For example, an SDR is expected for morphine analgesia pilocarpine drops for mydriasis or adrenaline subcutaneous administration to increase blood pressure and heart rate. Similarly, levodopa has a plasma half-life of 6090 min and possesses a very powerful antiparkinsonian effect. So, it is not surprising that such stimulation of the dopaminergic system in PD leads to a change in response that manifests as wearing-off and other motor complications. In this section, we review the main pathophysiological mechanisms implicated in the origin of motor fluctuations in PD.
Recommended Reading: Ms And Parkinson’s Disease
Clinical Features Of Wearing
As soon as levodopa was introduced into clinical practice for the treatment of PD, it was recognized that the therapeutic response consists of at least two components : the short-duration response , which provides an improvement in motor disability that lasts a few hours after the administration of single doses of levodopa, and the long-duration response , which is a sustained antiparkinsonian effect derived from prolonged administration of levodopa that has been shown to last for up to 2 weeks after cessation of drug treatment . Importantly, both types of response are present from the initiation of therapy, although the SDR is largely unnoticed in the beginning as the LDR masks it .
For many years, the development of wearing-off has been mainly attributed to a shortening of the SDR over time as a result of the progressive reduction in the ability of the nigrostriatal neurons to synthesize and to store dopamine formed from exogenous levodopa . However, a number of studies have shown that the magnitude of the SDR and modifications of the LDR during the course of PD also have a critical role in the development of symptom re-emergence .
Study Limitations And Future Research
The study authors recognize that self-reporting may reduce the accuracy of the figures and that the observational nature of our study makes it impossible to draw a conclusion regarding causality. They accept that these are limitations of the study.
Speaking about future research, Dr. Tsukita told MNT: The next step is certainly a with a long-term intervention of exercise. There is a tendency to emphasize high-intensity exercise in the field of PD exercise, but our study strongly indicates that exercise may modify the long-term course of PD even in small amounts if sustained. Therefore, future RCTs should focus on sustained exercise as well, for example, by using motivational apps.
APDA offers free booklets, presentations, and videos to guide those with PD through specifically designed exercises that people can perform safely at home.
Also Check: Emory Parkinson’s Disease Research