How Is It Treated And Is There A Cure
For now, Parkinsons disease is not curable, but there are multiple ways to manage its symptoms. The treatments can also vary from person to person, depending on their specific symptoms and how well certain treatments work. Medications are the primary way to treat this condition.
A secondary treatment option is a surgery to implant a device that will deliver a mild electrical current to part of your brain . There are also some experimental options, such as stem cell-based treatments, but their availability often varies, and many aren’t an option for people with Parkinsons disease.
Causes Of Parkinson’s Disease
Parkinson’s disease is caused by a loss of nerve cells in part of the brain called the substantia nigra. This leads to a reduction in a chemical called dopamine in the brain.
Dopamine plays a vital role in regulating the movement of the body. A reduction in dopamine is responsible for many of the symptoms of Parkinson’s disease.
Exactly what causes the loss of nerve cells is unclear. Most experts think that a combination of genetic and environmental factors is responsible.
Is Parkinson’s Disease An Autoimmune Disease
Synopsis:New study of Parkinsons disease proposes neurons may be mistaken for foreign invaders and killed by the human immune system.
The cause of neuronal death in Parkinson’s disease is still unknown, but a new study proposes that neurons may be mistaken for foreign invaders and killed by the person’s own immune system, similar to the way autoimmune diseases like type I diabetes, celiac disease, and multiple sclerosis attack the body’s cells. The study was published April 16, 2014, in Nature Communications.
Also Check: Theracycle For Parkinson’s Disease
Parkinsons: Autoimmune Attack May Start Years Before Diagnosis
A new study adds to evidence that autoimmunity plays a role in the development of Parkinsons disease. The research also offers hope that early preventive treatment could offset the damage.
Parkinsons disease is a chronic, progressive disorder. Its characteristics tend to include tremor, rigidity, slowness of movement, and impaired balance.
Around 1 million people in the United States and 10 million people throughout the world have the disease.
Parkinsons results from a loss of nerve cells in a part of the brain called the substantia nigra. These cells produce dopamine, a chemical messenger, or neurotransmitter, involved in controlling movement.
Most people with Parkinsons are older than 50 when they receive the diagnosis, but some develop motor symptoms, involving problems with muscle control, at an earlier age.
Years before motor symptoms arise, other symptoms of Parkinsons can appear, including a reduced sense of smell, constipation, mood changes, and REM sleep behavior disorder, which involves physically acting out dreams.
The existence of these prediagnostic symptoms suggests that damage to dopamine-producing nerve cells begins long before the person experiences trouble with movement.
A new study spearheaded by researchers from the La Jolla Institute for Immunology , in California adds to evidence that the immune system may be responsible for the damage to nerve cells.
Box 2 Genes Risk Factors And Modifiers In Pd Converge On The Lysosome
Many Parkinson disease -associated genes identified by familial Mendelian inheritance patterns have roles in lysosomal function. Candidate risk genes for PD, such as TMEM175, TMEM230, CTSB, VPS13C, GBA, SYT11 and ATP6V0A1, are also linked with lysosomal functions,, with a significant proportion of rare lysosomal disorder gene variants associated with PD risk. These genes have been implicated in various aspects of autophagy and lysosomal function, including in regulation of lysosomal pH, vesicular trafficking, autophagosome biogenesis, phagocytosis and cargo-specific autophagy,, . Such cellular processes are crucial for efficient immune cell function but detailed mechanistic insight into the roles of these PD-associated genes in inflammation remains lacking.
The involvement of aberrant microglial phagocytosis in PD is supported by the fact that microglia uptake and remove dopaminergic neuronal cell debris in vivo and can engulf -synuclein possibly via Toll-like receptor 4 ,. Extracellular -synuclein can directly activate microglia, with -synuclein fibrils and mutations associated with early-onset PD leading to the most robust levels of immune activation in BV2 microglial-like cells. Furthermore, PD-associated genes have been shown to regulate phagocytosis in microglia,, and macrophages,,. However, the precise contribution of microglial to PD is still unknown.
ER, endoplasmic reticulum GBA, glucocerebrosidase PINK1, PTEN-induced kinase 1 ROS, reactive oxygen species.
Recommended Reading: Does David Brooks Have Parkinson’s
Innate And Adaptive Immunity In Pd
Several genes implicated in familial forms of PD are associated with mechanisms linked to immunity. They are involved in immune responses, endocytosis, lysosomal functions, autophagy, and mitochondrial biology. Some of them, such as LRRK2 , PRKN , PINK1 , and DJ-1 have been particularly investigated in addition to SNCA. Several specific
In Vitro Studies Of Ipsc
A recent in vitro study using induced-pluripotent stem cells -derived midbrain neurons and T cells from PD patients was the first to show that PD patient-derived T cells can kill dopamine neurons directly. Sommer et al. determined that PD patients contain significantly higher Th17 cells than healthy controls . The PD patient-derived Th17 cells exerted cytotoxic effects on neurons by releasing IL-17A, a cytokine detected by IL-17R expressed on neurons . The iPSC in vitro cultures lacked glia, which express MHC-II and can potentially interact with Th17 cells. In addition, T cells were activated non-specifically, and so the antigen specificity of Th17 cells remains unclear . While the study indicates that PD-derived T cells can directly kill dopaminergic neurons, the omission of professional antigen presenting cells, antigenicity, and neuronal specificity in the cultures in this initial study overlooks the role of multiple relevant in vivo factors important for disease progression. Moreover, the mode of action that garners specific vulnerability of dopaminergic neurons and avoids unaffected neurons was not resolved in this study. Nevertheless, the study indicates Th17 cells may participate in PD-related neuronal death.
Also Check: Early Onset Parkinson’s Tremor
What Causes The Condition
Although there are several recognized risk factors for Parkinsons disease, such as exposure to pesticides, for now, the only confirmed causes of Parkinsons disease are genetic. When Parkinsons disease isnt genetic, experts classify it as idiopathic . That means they dont know exactly why it happens.
Many conditions look like Parkinson’s disease but are instead parkinsonism from a specific cause like some psychiatric medications.
Familial Parkinsons disease
Parkinsons disease can have a familial cause, which means you can inherit it from one or both of your parents. However, this only makes up about 10% of all cases.
Experts have linked at least seven different genes to Parkinson’s disease. They’ve linked three of those to early-onset of the condition . Some genetic mutations also cause unique, distinguishing features.
Idiopathic Parkinsons disease
Experts believe idiopathic Parkinsons disease happens because of problems with how your body uses a protein called -synuclein . Proteins are chemical molecules that have a very specific shape. When some proteins dont have the correct shape a problem known as protein misfolding your body cant use them and can’t break them down.
With nowhere to go, the proteins build up in various places or in certain cells . The buildup of these Lewy bodies causes toxic effects and cell damage.
Induced Parkinsonism
The possible causes are:
Introduction: Parkinson’s Disease And Inflammation
Multiple studies have highlighted an association between sustained inflammation and a number of neurodegenerative diseases including Alzheimer’s disease , Parkinson’s disease , amyotropic lateral sclerosis , and frontal temporal dementia . The role of inflammation in the pathogenesis of these disorders, however, remains undetermined. Here, we focus on the inflammatory features in PD, the most common movement disorder, affecting more than 10 million people worldwide . PD patients manifest motor symptoms including bradykinesia, rest tremor, muscular rigidity, and postural and gait impairment, as well as non-motor symptoms . Non-motor symptoms include mood disorders, cognitive impairments, and autonomic dysfunction, such as orthostatic hypotension and constipation . While alleles of many genes are associated with the disorder, PD remains largely a sporadic disorder associated with older age and various genetic and environmental risk factors .
PD is diagnosed from motor symptoms , but non-motor symptoms are often manifest during a prolonged prodromal phase as much as 20 years prior to the onset of the motor features . These prodromal non-motor symptoms include constipation, rapid eye movement sleep behavior disorder, depression, anosmia, and excessive daytime sleepiness . The sensitivity and the specificity of these non-motor symptoms limits their utility in predicting the development of PD .
You May Like: I Have Parkinson’s Disease
Is Parkinsons An Autoimmune Disease
Experts still do not know the root of Parkinsons disease . Science has found that this movement disorder is caused by a combination of environmental and genetic factors. Most people with PD first develop the disease after age 60.
Despite uncertainties regarding the cause of PD, research over the last several years points to dysfunctions in the immune system. Its still not clear whether PD can be considered an autoimmune condition.
Pathogenic Protein Function In Autoimmunity
As discussed above, molecular mimicry and cross immunoreactions are two of the primary mechanisms through which autoimmunity is triggered. Molecular mimicry between herpes simplex virus 1 and human -syn was detected in PD patients in 2016. HSV1 infection could enhance the development of autoimmunity because autoreactive antibodies induced by HSV1 have the same response to the human -syn homologous peptide bound to the membrane of DNs and lead to DN destruction . These results also support the assumption that -syn participates in autoimmunity involved in the pathological progression of PD.
Recommended Reading: Parkinson’s Disease Research Foundation
What Can I Expect If I Have This Condition
Parkinsons disease is a degenerative condition, meaning the effects on your brain get worse over time. However, this condition usually takes time to get worse. Most people have a normal life span with this condition.
You’ll need little to no help in the earlier stages and can keep living independently. As the effects worsen, youll need medication to limit how the symptoms affect you. Most medications, especially levodopa, are moderately or even very effective once your provider finds the minimum dose you need to treat your symptoms.
Most of the effects and symptoms are manageable with treatment, but the treatments become less effective and more complicated over time. Living independently will also become more and more difficult as the disease worsens.
How long does Parkinsons disease last?
Parkinsons disease isnt curable, which means its a permanent, life-long condition.
Whats the outlook for Parkinsons disease?
Parkinson’s disease isn’t fatal, but the symptoms and effects are often contributing factors to death. The average life expectancy for Parkinson’s disease in 1967 was a little under 10 years. Since then, the average life expectancy has increased by about 55%, rising to more than 14.5 years. That, combined with the fact that Parkinson’s diagnosis is much more likely after age 60, means this condition doesn’t often affect your life expectancy by more than a few years .
Parkinsons Disease And Autoimmunity
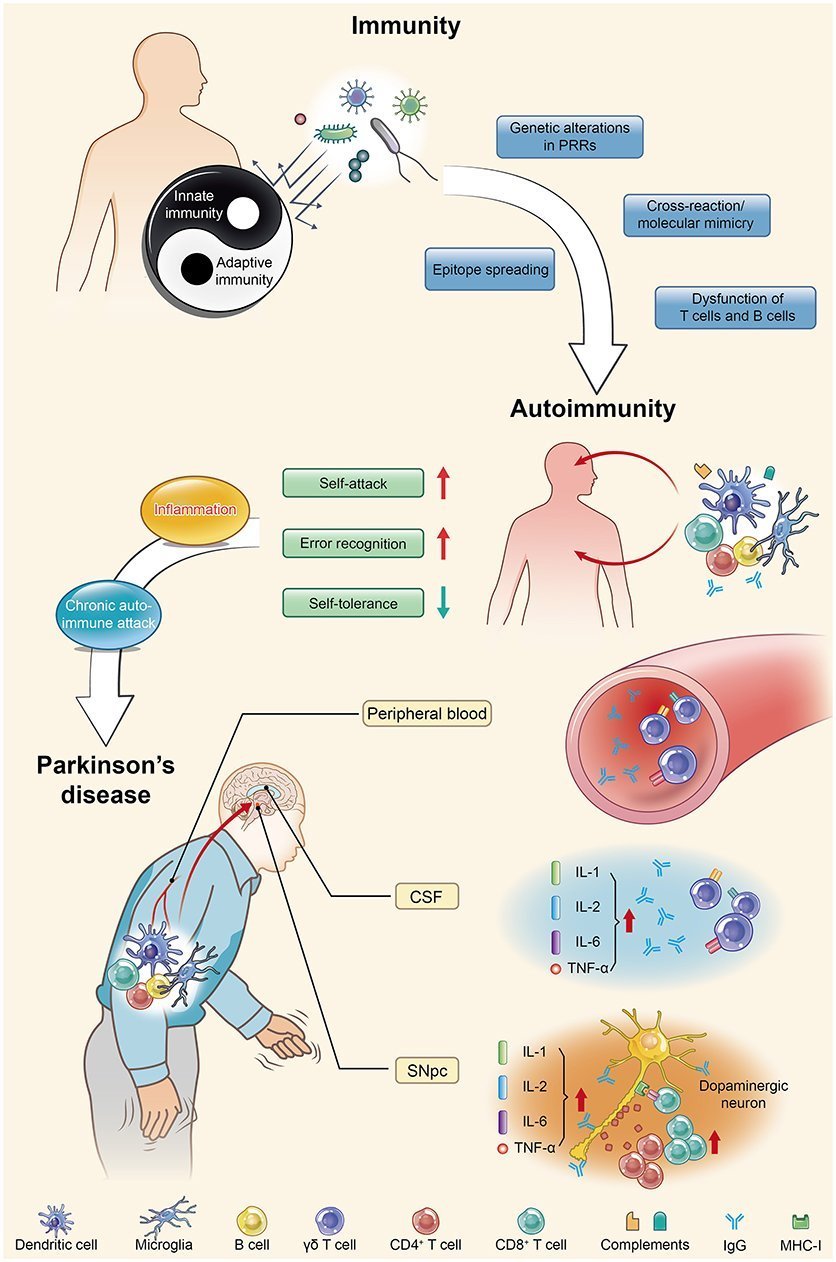
This autoimmune component of PD may only be one piece of a larger puzzle. It has long been suspected that immune alterations are an important part of the development of PD, says Maureen Leehey, MD, professor of neurobiology at the University of Colorado School of Medicine in Aurora, CO. She notes previous research that has linked ibuprofen use in mid-life with lower PD risk. And studies have shown that in the early stages, but not the late stages, there is activation of immune fighting cells in the brains of persons with PD, Dr. Leehey says. I think that immune alterations are an important part of the progression of central nervous system damage in PD.
This isnt the first research being done on Parkinsons and autoimmunity there have been multiple studies in this vein in recent years. In 2017, the same researchers from La Jolla and Columbia University identified a specific protein that drives the T cell response in early Parkinsons disease. Further work has suggested that Parkinsons could be triggered by bacterial infection.
Paredes explains that changes in the can cause chronic inflammation that might influence Parkinsons symptoms. An April 2020 study in Nature Genetics theorized that Parkinsons may start in the gut, due to evidence that neurons in the gut are directly associated with the onset of disease.
You May Like: Can You Drive With Parkinson’s Disease
No One Definitive Cause Of Parkinsons
There are no biomarkers or objective screening tests that indicate one has Parkinsons. That said, medical experts have shown that a constellation of factors are linked to it.
Parkinsons causes are likely a blend of genetics and environmental or other unknown factors. About 10 to 20 percent of Parkinsons disease cases are linked to a genetic cause, says Ted Dawson, M.D., Ph.D., director of the Institute for Cell Engineering at Johns Hopkins. The types are either autosomal dominant or autosomal recessive .
But that leaves the majority of Parkinsons cases as idiopathic, which means unknown. We think its probably a combination of environmental exposure to toxins or pesticides and your genetic makeup, says Dawson.
Age. The biggest risk factor for developing Parkinsons is advancing age. The average age of onset is 60.
Gender. Men are more likely to develop Parkinsons disease than women.
Genetics. Individuals with a parent or sibling who is affected have approximately two times the chance of developing Parkinsons. Theres been an enormous amount of new information about genetics and new genes identified over the past 10 or 15 years that have opened up a greater understanding of the disease, says Dawson.
What Tests Will Be Done To Diagnose This Condition
When healthcare providers suspect Parkinsons disease or need to rule out other conditions, various imaging and diagnostic tests are possible. These include:
- Blood tests .
- Positron emission tomography scan.
New lab tests are possible
Researchers have found possible ways to test for possible indicators or Parkinsons disease. Both of these new tests involve the alpha-synuclein protein but test for it in new, unusual ways. While these tests cant tell you what conditions you have because of misfolded alpha-synuclein proteins, that information can still help your provider make a diagnosis.
The two tests use the following methods.
- Spinal tap. One of these tests looks for misfolded alpha-synuclein proteins in cerebrospinal fluid, which is the fluid that surrounds your brain and spinal cord. This test involves a spinal tap , where a healthcare provider inserts a needle into your spinal canal to collect some cerebrospinal fluid for testing.
- Skin biopsy. Another possible test involves a biopsy of surface nerve tissue. A biopsy includes collecting a small sample of your skin, including the nerves in the skin. The samples come from a spot on your back and two spots on your leg. Analyzing the samples can help determine if your alpha-synuclein has a certain kind of malfunction that could increase the risk of developing Parkinsons disease.
Also Check: Are Hallucinations A Symptom Of Parkinson’s
Inflammatory Bowel Disease Irritable Bowel Syndrome And Parkinsons Disease
Gastrointestinal symptoms are frequently found in PD and may exist decades before motor symptom onset. Bidirectional communication between the central nervous system and enteric nervous system has been proposed as a possible link between the intestinal environment and PD. Postmortem studies showing the presence of aggregated alpha-synuclein in the ENS, particularly the appendix . In support of its role, patients with appendectomies have been reported to have a delayed age of PD onset . Intestinal dysbiosis may also affect alpha-synuclein aggregation and lead to an excessive inflammatory response and potentially contribute to disease onset and progression. The gut microbiome and its effect on PD is covered by others in this special issue of the Journal of Parkinsons Disease.
Some of the above associations seemed to be age dependent. In one meta-analysis patients with onset of IBD over age of 60 had a higher risk of PD . By contrast, another study demonstrated a higher risk of PD in CD patients only in individuals with age at onset younger than 60 years . The incidence of IBD among PD patients has also been controversial. A US study using Medicare data showed a lower risk of both CD and UC in PD patients compared to non-PD controls . This discrepancy could be due to the gap in peak incidence age between diseases. It may also be that typical symptom of IBD may be masked by other gastrointestinal issues in PD, such as delayed gastric emptying and constipation.
What Are The Early Warning Signs Of Parkinson’s Disease
Parkinsons warning signs can be motor symptoms like slow movements, tremors or stiffness. However, they can also be non-motor symptoms. Many of the possible non-motor symptoms can appear years or even decades ahead of motor symptoms. However, non-motor symptoms can also be vague, making it difficult to connect them to Parkinson’s disease.
Non-motor symptoms that might be early warning signs include:
- Sleep problems such as periodic limb movement disorder , rapid eye movement behavior disorder and restless legs syndrome.
Don’t Miss: Does Sam Waterston Have Parkinson
Inflammation In Pd Pathogenesis And Progression
Considering age is the greatest risk factor for many neurodegenerative diseases, the ageing of the immune system is the most underappreciated and understudied contributing factor in the neurodegeneration field. Immunosenescence is characterized by two primary features, namely an age-acquired immunodeficiency and . Inflammageing is characterized by excess low-level production of circulating inflammatory mediators, or cytokines most notably C-reactive protein , IL-6 and tumour necrosis factor from chronically stimulated innate and adaptive immune cells,,. Both the innate and adaptive immune systems lose competence with ageing and are also notably altered in PD, .
Fig. 2: Inflammatory manifestations in PD.
The figure highlights inflammatory manifestations that have been identified in patients with Parkinson disease . Intestinal dysbiosis and inflammation , increases in levels of circulating pro-inflammatory cytokines , innate and adaptive immune cell activation and changes in frequency , bloodbrain barrier permeability and peripheral immune cell infiltration of the central nervous system and neuroinflammation are hallmarks of a pro-inflammatory immune phenotype in PD. ROS, reactive oxygen species.