No One Definitive Cause Of Parkinsons
There are no biomarkers or objective screening tests that indicate one has Parkinsons. That said, medical experts have shown that a constellation of factors are linked to it.
Parkinsons causes are likely a blend of genetics and environmental or other unknown factors. About 10 to 20 percent of Parkinsons disease cases are linked to a genetic cause, says Ted Dawson, M.D., Ph.D., director of the Institute for Cell Engineering at Johns Hopkins. The types are either autosomal dominant or autosomal recessive .
But that leaves the majority of Parkinsons cases as idiopathic, which means unknown. We think its probably a combination of environmental exposure to toxins or pesticides and your genetic makeup, says Dawson.
Age. The biggest risk factor for developing Parkinsons is advancing age. The average age of onset is 60.
Gender. Men are more likely to develop Parkinsons disease than women.
Genetics. Individuals with a parent or sibling who is affected have approximately two times the chance of developing Parkinsons. Theres been an enormous amount of new information about genetics and new genes identified over the past 10 or 15 years that have opened up a greater understanding of the disease, says Dawson.
Measurement Of Global Antibody Plasma Levels
To assess whether the global humoral immune system encompassed the antigen-specific IgG subclass and IgM responses, we measured the global concentrations of IgG subclass and IgM antibodies in plasma samples. We have used the commercially available Ready-SET-Go!® ELISA kits following the manufacturer’s instructions. In brief, plasma samples were diluted in the assay buffers supplied with the kits according to manufacturer-recommended: IgG1 ELISA kit: 1:2,000 IgG2 ELISA kit: 1:500,000 IgG3 ELISA kit: 1:40,000 IgG4 ELISA kit: 1:1,000 IgG-total ELISA kit: 1:500,000 , and IgM 1:20,000 .
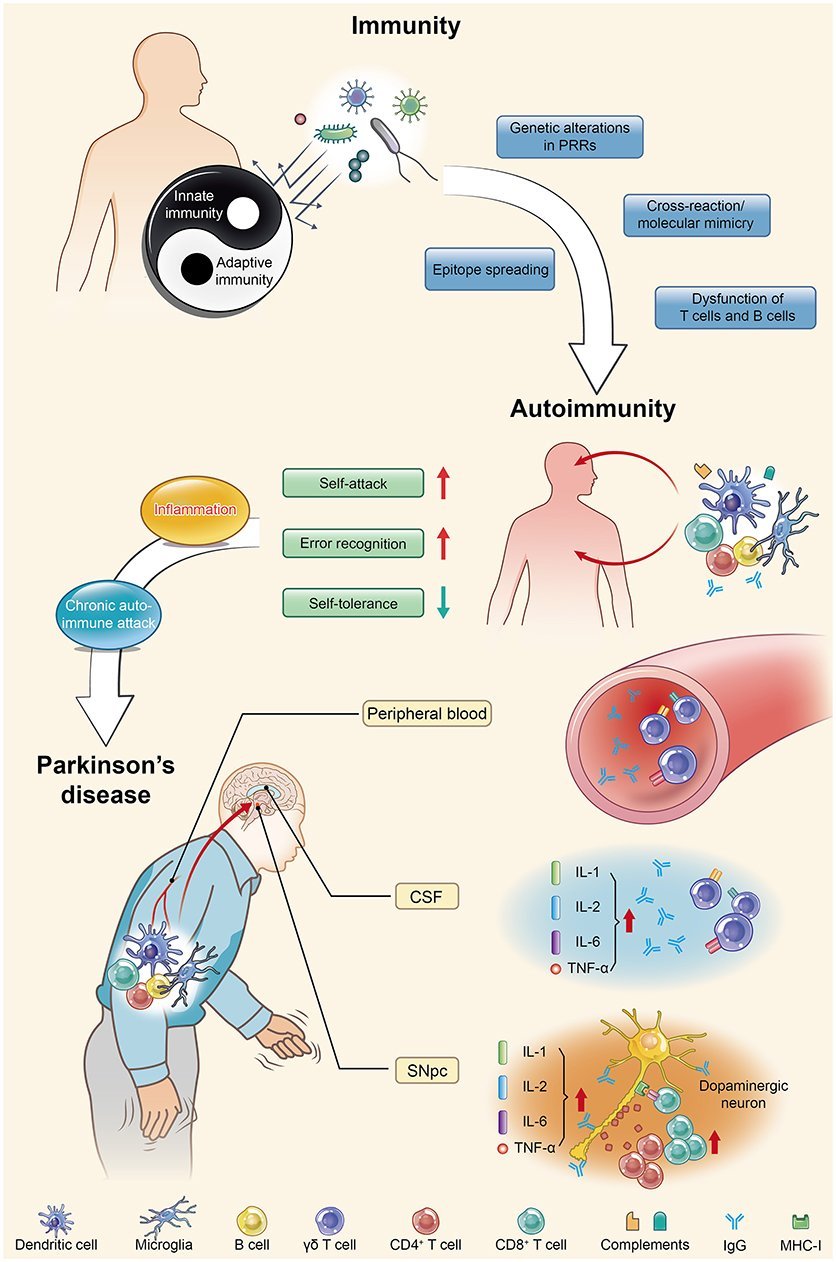
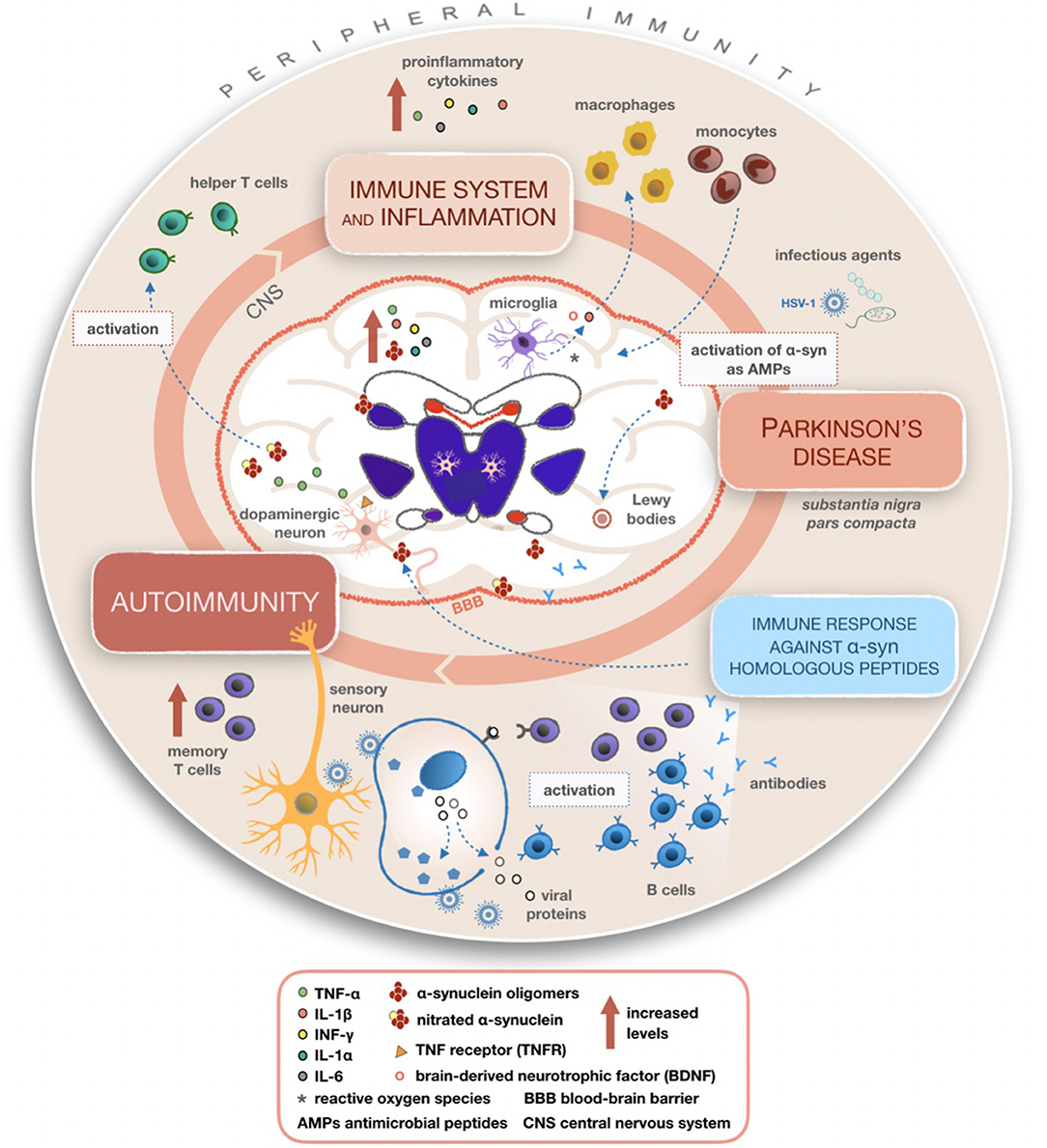
Introduction: Parkinsons Disease And Inflammation
Multiple studies have highlighted an association between sustained inflammation and a number of neurodegenerative diseases including Alzheimers disease , Parkinsons disease , amyotropic lateral sclerosis , and frontal temporal dementia . The role of inflammation in the pathogenesis of these disorders, however, remains undetermined. Here, we focus on the inflammatory features in PD, the most common movement disorder, affecting more than 10 million people worldwide . PD patients manifest motor symptoms including bradykinesia, rest tremor, muscular rigidity, and postural and gait impairment, as well as non-motor symptoms . Non-motor symptoms include mood disorders, cognitive impairments, and autonomic dysfunction, such as orthostatic hypotension and constipation . While alleles of many genes are associated with the disorder, PD remains largely a sporadic disorder associated with older age and various genetic and environmental risk factors .
PD is diagnosed from motor symptoms , but non-motor symptoms are often manifest during a prolonged prodromal phase as much as 20 years prior to the onset of the motor features . These prodromal non-motor symptoms include constipation, rapid eye movement sleep behavior disorder, depression, anosmia, and excessive daytime sleepiness . The sensitivity and the specificity of these non-motor symptoms limits their utility in predicting the development of PD .
You May Like: I Have Parkinsons Disease
Recommended Reading: Myrbetriq And Parkinson’s Disease
Is There A Link
Some people have MS and Parkinsonâs.
Research suggests that the damage that MS causes to your brain can lead some people to develop Parkinsonâs later on.
If you have MS, your immune system triggers ongoing inflammation. This can create lesions in your brain that cause Parkinsonâs disease. If lesions form in certain spots in your brain, they can affect how it makes dopamine.
Is Parkinsons Disease An Autoimmune Disease
Synopsis:New study of Parkinsons disease proposes neurons may be mistaken for foreign invaders and killed by the human immune system.
The cause of neuronal death in Parkinsons disease is still unknown, but a new study proposes that neurons may be mistaken for foreign invaders and killed by the persons own immune system, similar to the way autoimmune diseases like type I diabetes, celiac disease, and multiple sclerosis attack the bodys cells. The study was published April 16, 2014, in Nature Communications.
Also Check: Theracycle For Parkinsons Disease
Read Also: Lion’s Mane Parkinson’s
Clinical Features And Autoimmunity In Pd
-Synuclein participates in autoimmunity and is involved in the pathological progression of PD. -Syn, as the main disease-causing protein, first appears in the gut and is related to gut dysbiosis, which disturbs the intestinal immune system, leading to one of the main non-motor symptoms of PD: constipation. Then, this protein transmits to the dorsal motor nucleus through the vagus nerve and acts as a self-antigen targeted by effector T cells, B cells, and microglia. This autoimmunity attack finally results in the damage and death of DNs. SIBO, small intestinal bacterial overgrowth.
Causes Of Parkinsons Disease
Parkinsons disease is caused by a loss of nerve cells in part of the brain called the substantia nigra. This leads to a reduction in a chemical called dopamine in the brain.
Dopamine plays a vital role in regulating the movement of the body. A reduction in dopamine is responsible for many of the symptoms of Parkinsons disease.
Exactly what causes the loss of nerve cells is unclear. Most experts think that a combination of genetic and environmental factors is responsible.
Don’t Miss: Medication For Early Onset Parkinson’s
Future Diagnosis And Treatment Of Parkinsons
If these theories are proved true, they may affect the way PD is diagnosed and treated in the future. Blood samples can provide evidence of autoimmune responses against alpha-synuclein in people with PD. This finding could pave the way for blood tests to diagnose PD and may be especially helpful in the early stages of Parkinsons.
How The Study Worked
The researchers compared the activity of genes in memory T cells from people with Parkinsons and those from healthy controls matched for their age.
When they focused on patients whose T cells reacted to alpha-synuclein, they discovered a range of genes with different activity levels than controls.
Among these were genes previously linked to Parkinsons, including some that are involved in oxidative stress and inflammation.
The genetic signature of Parkinsons in these cells also included a gene called LRRK2, which is 1 of 2 genes most commonly linked to the familial type of Parkinsons that runs in families.
The gene is known to be active in neurons, where it plays a role in the disease process, but this is the first time scientists have found it to be active in T cells.
The association of LRRK2 with PD has been known for a long time, said Professor Sette.
However, the genetic signature includes several other genes not previously linked to the disease.
What we are most excited about is the large number of new and novel potential targets that the approach has uncovered, Prof. Sette told MNT.
It may be possible to delay or halt the progression of the disease by targeting these genes in the early stages before motor symptoms develop.
Don’t Miss: Knee Replacement Surgery Recovery And Parkinson’s
What Causes Parkinsons Disease
Parkinsons disease is a chronic, progressive neurological disease that currently affects about 1 million Americans. Parkinsons disease involves a small, dark-tinged portion of the brain called the substantia nigra. This is where you produce most of the dopamine your brain uses. Dopamine is the chemical messenger that transmits messages between nerves that control muscle movements as well as those involved in the brains pleasure and reward centers. As we age, its normal for cells in the substantia nigra to die. This process happens in most people at a very slow rate.
But for some people, the loss happens rapidly, which is the start of Parkinsons disease. When 50 to 60 percent of the cells are gone, you begin to see the symptoms of Parkinsons.
Investigating Parkinsons As An Autoimmune Disease
At the University of Montreal, Professor Louis-Eric Trudeau investigates the earliest potential causes of Parkinsons disease, at the cellular level. His project is funded by the Saucier-van Berkom Parkinson Quebec Research Fund with contributions from Parkinson Newfoundland & Labrador, in the amount of $49,748.00 for 1 year. He is exploring the possibility that Parkinsons is a form of autoimmune disease, caused when the immune system attacks the axon terminals in our brain cells. Those extremities release the chemical messengers that communicate with other cells, and damage to such terminals may disrupt the dopamine-producing brain cells that are key to Parkinsons.
The death of the brain cells that produce dopamine, the chemical messenger that signals other cells involved in motor control, triggers the symptoms of Parkinsons disease. But researchers still dont know exactly what causes those dopamine-producing cells to die.
Trudeau, a neuroscientist, investigates the possibility that an autoimmune attack on those dopamine cells is the culprit.
Trudeau and his immunologist colleague, Michel Desjardins, are studying the role of the portion of cells called axon terminals. These terminalsthe root-like extremities of cellsrelease the chemical messengers that send communication signals. Trudeau believes the death of these terminals, before the death of the dopamine cells themselves, is where the trouble starts.
Read Also: High Blood Pressure And Parkinson’s
Parkinsons: Autoimmune Attack May Start Years Before Diagnosis
A new study adds to evidence that autoimmunity plays a role in the development of Parkinsons disease. The research also offers hope that early preventive treatment could offset the damage.
Parkinsons disease is a chronic, progressive disorder. Its characteristics tend to include tremor, rigidity, slowness of movement, and impaired balance.
Around 1 million people in the United States and 10 million people throughout the world have the disease.
Parkinsons results from a loss of nerve cells in a part of the brain called the substantia nigra. These cells produce dopamine, a chemical messenger, or neurotransmitter, involved in controlling movement.
Most people with Parkinsons are older than 50 when they receive the diagnosis, but some develop motor symptoms, involving problems with muscle control, at an earlier age.
Years before motor symptoms arise, other symptoms of Parkinsons can appear, including a reduced sense of smell, constipation, mood changes, and REM sleep behavior disorder, which involves physically acting out dreams.
The existence of these prediagnostic symptoms suggests that damage to dopamine-producing nerve cells begins long before the person experiences trouble with movement.
A new study spearheaded by researchers from the La Jolla Institute for Immunology , in California adds to evidence that the immune system may be responsible for the damage to nerve cells.
Is Parkinsons An Autoimmune Disease
The cause of neuronal death in Parkinsons disease is still unknown, but a new study proposes that neurons may be mistaken for foreign invaders and killed by the persons own immune system, similar to the way autoimmune diseases like type I diabetes, celiac disease, and multiple sclerosis attack the bodys cells. The study was published April 16, 2014, in Nature Communications.
Don’t Miss: Enfermedad De Parkinson En Español
Genetic Regulation Of Autoimmunity In Pd
In addition to these observations, DJ-1 has also been reported to affect the development of natural Tregs and induced Tregs . Mature Tregs with normal function, which modulate not only adaptive immunity but also innate immunity, are pivotal for maintaining thymic function, peripheral immune self-tolerance and immune system homeostasis. nTregs are generated in the thymus, while iTregs are derived from naïve CD4+ T cells encountering antigens in the peripheral organs. Both cell types are generally immunosuppressive through the suppression or downregulation of effector T cell proliferation . Their self-check function successfully prevents excessive effector cell reactions. On the other hand, the abnormal proliferation of both types of Tregs leads to the failure of self-/non-self-discrimination, resulting in autoimmune disease . Evidence reported by Singh et al. has demonstrated that DJ-1, one of the most classical key players responsible for PD pathogenesis, is strongly linked with neuroimmunology and multiple autoimmune responses in PD . In addition, DJ-1-deficient animal models have shown compromised iTreg induction, cell cycle progression, and cell survival and proliferation. DJ-1/ iTregs are more proliferative, more susceptible to cell death signals and deficient in cell division compared with wild type counterparts, as analyzed by flow cytometry and Western blotting.
Talk With Others Who Understand
MyParkinsonsTeam is the social network for people with Parkinsons disease and their loved ones. On MyParkinsonsTeam, more than 88,000 members come together to ask questions, give advice, and share their stories with others who understand life with Parkinsons disease.
Do you still have questions about what causes Parkinsons? Share your experience in the comments below, or start a conversation by posting on your Activities page.
Read Also: Is There A Surgery For Parkinson’s
The Neuromuscular & Movement Disorders Division Focuses On These Disorders:
Peripheral neuropathy affects 20 million people in the U.S. Initial symptoms include numbness, tingling and pain that often interferes with sleep and function. The hands and feet are typically affected. While the causes of neuropathy are numerous, most commonly neuropathy is due to diabetes or prediabetes.
Myopathies are diseases of skeletal muscle in which there is typically proximal muscle weakness without sensory loss. Some myopathies are autoimmune, toxic or due to other acquired causes while others are hereditary. Myopathies are due to a primary structural or functional impairment of muscle including genetic metabolic disorders and disorders of ion channels in membranes that mediate the electrical signals responsible for muscle contraction. Diseases of muscle structural proteins or muscular dystrophies are caused by mutations in genes encoding for essential membrane or extracellular matrix proteins, sarcomeric components or nuclear envelope molecules. Disorders of muscles can be categorized and subdivided so that it is generally possible to characterize a particular myopathy based on its distinctive features.
Multiple System Atrophy is a rare form of Parkinsonism resembling Parkinson’s disease but with more rapid disease progression. Currently there are no treatments for Multiple System Atrophy.
- IND research: Our neuromuscular research database includes 4,500 patients.
Thanks For Signing Up
We are proud to have you as a part of our community. To ensure you receive the latest Parkinsons news, research updates and more, please check your email for a message from us. If you do not see our email, it may be in your spam folder. Just mark as not spam and you should receive our emails as expected.
You May Like: What Other Diseases Mimic Parkinson’s
Changes In T Cell Subpopulations And Cytokines
Consistent with the systemic view that PD involves multiple systems and tissues, several studies have shown general alterations in cytokines and immune cell populations.
Proinflammatory cytokines are elevated in the blood of PD patients, including increased levels of IL-2 6 8 , MCP-1 , MIP-1 , RANTES , TNF , and IFN . Increased levels of proinflammatory cytokines and chemokines are indicative of an immune system responding to tissue damage and/or foreign molecules. The levels of cytokines and chemokines correlate with the clinical stage of the disease, highlighting a role for peripheral inflammation in PD progression . Altered T cells populations can also contribute to the changes in circulating cytokines. Th1 and TH17+ CD4+ cells can contribute to the increased levels of IFN, TNF, and IL-17 .
Nabs Against Pathological Antigens
Our model described significant differences in levels of -syn reactive antibodies for all IgG subclasses and IgMs and IgM , whereas the total levels of anti–syn IgG NAbs were similar in all groups, with neither age nor sex being significantly confounding factors. The relative levels of anti–syn IgG1 were higher in MSA compared to PD . Similarly, the anti–syn IgG3 levels were higher in MSA compared to PD . Anti–syn IgG2 levels described by disease group were higher in PD compared to controls , as were the described anti–syn IgG4 levels by group , whereas anti–syn IgG4 was lower in PD compared to MSA and controls . Further, anti–syn IgM levels were lower in both MSA and PD as compared to controls described by group .
Figure 1. Plasma -synuclein naturally occurring autoantibody levels. Distribution of relative anti–synuclein naturally occurring autoantibody plasma levels in patients with Multiple System Atrophy divided into subtypes , patients with Parkinson’s disease , and controls . ELISA relative ODs of anti–synuclein total IgG, IgG1, IgG2, IgG3, IgG4, and IgM autoantibodies. Dot plots show relative ODs with mean values ± SEM. Differences were tested using one-way ANOVA and Tukey’s post hoc test adjusted for age and sex.
Recommended Reading: Parkinson’s Disease And Cough Medicine
Parkinsons An Autoimmune Disease
Discovering the cause or etiology of diseases such as Parkinsons is essential to finding a treatment to prevent it. There are more and more advances in this field. And one of the latest ones is that Parkinsons could be an autoimmune disease.
Índice
Aggressive Immune Cells Aggravate Parkinson’s Disease
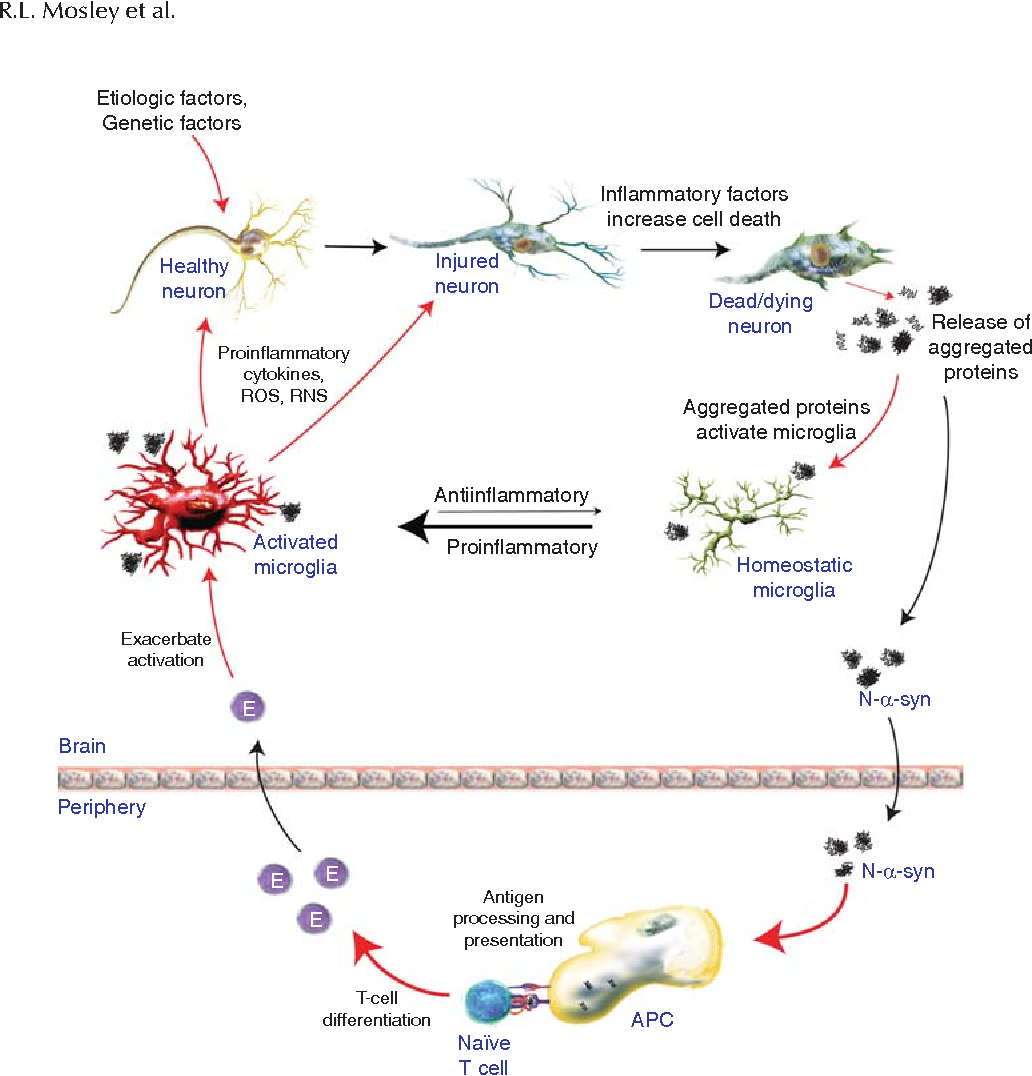
Parkinsons disease, formerly also referred to as shaking palsy, is one of the most frequent disorders affecting movement and the nervous system. Medical researchers at Friedrich-Alexander-Universität Erlangen-Nürnberg have come across a possible cause of the disease in the patients immune system. The scientists have published their research findings in the magazine Cell Stem Cell .
Currently, approximately 4.1 million people suffer from Parkinsons disease throughout the globe, in Germany alone more than 300,000 people are affected. Typical symptoms of the disease are slowness of movement, rigidity, frequent shaking and an increasingly stooped posture. The cause is the continuous death of nerve cells in the brain, which produce the messenger substance dopamine.
Scientists are working to gain insights into the mechanisms which lead to the loss of nerve cells that produce dopamine. Until now, little has been known about whether human immune cells have an important role to play in Parkinsons disease. The stem cell researchers Dr. Annika Sommer, Dr. Iryna Prots and Prof. Dr. Beate Winner from FAU and their team have made a major leap forward in research into this aspect of the disease. The scientists from Erlangen were able to prove that in Parkinsons disease immune cells from the immune system, so-called t-cells, attack and kill nerve cells which produce dopamine in the midbrain.
Contact:
Recommended Reading: Can Thyroid Problems Mimic Parkinson’s
Potential New Treatment Strategies For Parkinsons
This new finding provides additional knowledge and understanding of the disease processes that are present in PD and opens the door for using immunotherapies, drugs that suppress the abnormal immune response seen in autoimmune disorders. Additional research is needed to understand the molecular steps that occur in PD and the immune response, but researchers are hopeful that an immunotherapy strategy could help to prevent or lessen worsening symptoms in people with PD.1,2