What Medications Are Similar
Like Inbrija, Apokyn is another PD rescue or on-demand medication used to treat off episodes.
There are a number of differences between Inbrija and Apokyn, though. For example, while Inbrija is inhaled, Apokyn is taken sublingually or subcutaneously .
The drugs also differ in their side effect profiles and drug interactions. Apokyn further requires a titration process and medical supervision to determine the right dose.
Abstractformulae Display: Mathematical Formulae Have Been Encoded As Mathml And Are Displayed In This Html Version Using Mathjax In Order To Improve Their Display Uncheck The Box To Turn Mathjax Off This Feature Requires Javascript Click On A Formula To Zoom
ABSTRACT
Introduction: The most widely used pharmacological treatment for Parkinsons disease is levodopa, the precursor for dopamine formation in the brain. Over time, the effectiveness of levodopa declines, and patients experience motor fluctuations, or OFF periods. A levodopa formulation administered via a capsule-based oral inhaler provides a new delivery mechanism for levodopa that provides rapid relief of OFF periods.
Areas covered: CVT-301 is a dry powder formulation designed to supply levodopa to the systemic circulation via pulmonary absorption. The technology, pharmacokinetics, efficacy, and safety data of this formulation are presented.
Expert opinion: Oral inhalation is a novel method of administration for levodopa that bypasses the gastrointestinal tract, allowing levodopa to enter the systemic circulation rapidly and more reliably than oral medications. Gastrointestinal dysfunction, a common feature of Parkinsons disease, can lead to impaired absorption of oral medications. Pulmonary delivery rapidly elevates levodopa plasma concentrations to provide relief of OFF periods for patients receiving oral levodopa.
KEYWORDS:
Levodopa Inhalation Powder For Parkinsons Disease
Stuart Isaacson, MD: We have a lot of these new on-demand therapies that are not absorbed through the GI system that have become available. We have the levodopa inhalation powder. We have the sublingual apomorphine strip film. We also continue to have the injection subcutaneously of apomorphine thats been available for 15 years. Lets talk a little bit more about these therapies and see if we can understand maybe which ones we might choose here, or there, or what patient scenarios. Khash, do you want to tell us a little bit about your understanding and experiences with the levodopa inhalation powder, how it works, how its delivered, its mechanism of action and any advantages or disadvantages when you think about prescribing this drug?
Khashayar Dashtipor, MD: When I introduce my patients to this on-demand therapy, I go through the pros and cons of each one, such as inhaler levodopa. One big advantage is that its levodopa. We know that levodopa is one of the most potent medications and is the safest medication because of the adverse effect profile. That applies to when we use an inhaler as well.
One of the distinct adverse effects about inhaler levodopa comes from the mode of delivery of that. Coughing is one of its adverse effects. In comparison to other on-demand therapies, the rest of the adverse effects are definitely minimal, which is a big advantage.
Transcript edited for clarity.
Read Also: What Foods Should Be Avoided When Taking Levodopa
How To Take Inbrija
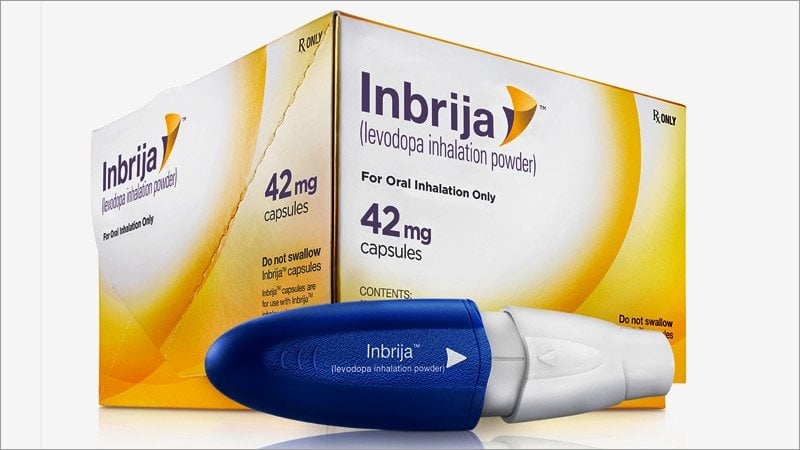
Inbrija comes in capsule form, with each capsule containing 42 milligrams of powdered levodopa. Each off episode is treated with two inhaled capsules for a total of 84 mg of levodopa.
According to the manufacturer, here are the steps for taking Inbrija:
It’s important to take an Inbrija dose as soon as you feel your PD symptoms return. Do take more than five doses of Inbrija in one day.
Do not swallow or open up any Inbrija capsules. In addition, be sure to only use your prescribed Inbrija capsules with your prescribed Inbrija inhaler. Do not use your Inbrija inhaler to take any other medications.
If you are feeling frustrated when taking Inbrija and/or are worried that you are not getting the medication into your body, don’t hesitate to reach out to your healthcare provider. The manufacturer of Inbrija also offers training and support from a nurse educator. They can be contacted at 1-888-887-3447. Pharmacists are a great and easily accessible resource for patients with questions and concerns, as well.
How Should I Use Levodopa Inhalation
Follow all directions on your prescription label and read all medication guides or instruction sheets. Use the medicine exactly as directed.
Levodopa inhalation is for use only in people who are currently taking carbidopa and levodopa. Do not stop taking your daily Parkinson’s medications or change your dosing schedule without your doctor’s advice.
For best results, use levodopa inhalation when an “off episode” begins . Do not use levodopa inhalation more than 5 times per day.
Read and carefully follow any Instructions for Use provided with your medicine. Ask your doctor or pharmacist if you do not understand these instructions.
Levodopa inhalation is a powder that comes with a special inhaler device and blister packs containing capsules of the medicine. You will load 2 capsules into the inhaler device each time you use the medicine. Pushing the handle of the device onto the mouthpiece will pierce the capsule and release the medicine into the inhaler.
Do not swallow a levodopa inhalation capsule. It is for use only in the Inbrija inhaler device.
This medicine can affect the results of certain medical tests. Tell any doctor who treats you that you are using levodopa inhalation.
Do not stop using levodopa inhalation suddenly after long-term regular use, or you could have unpleasant withdrawal symptoms . Ask your doctor how to safely stop using this medicine.
Throw away the device when your capsules run out. Always use the inhaler device that comes with each new prescription.
Read Also: Cleveland Clinic Parkinson’s Bicycle Study 2017
Potential For Inhaled Levodopa In Treatment Of Parkinsons Disease
Rescue therapy for Off periods in PD is an established therapeutic strategy, albeit one that does not address the underlying cause of motor fluctuations. Apomorphine hydrochloride injection is a currently available fast-acting dopamine agonist that offers onset of clinical benefit within 1020 minutes, but requires a subcutaneous injection to be administered and may be associated with nausea, hypotension, and development of subcutaneous nodules at injection sites.33 Premedication with an antiemetic is often required to avoid dose-related nausea and vomiting. Patients may experience difficulty manipulating the injection device in their Off state, requiring the assistance of a caregiver. A sublingual formulation of apomorphine hydrochloride is under clinical development,34 and may offer a more user-friendly delivery method. Adverse events can include dizziness, somnolence, and nausea.
Inbrija For Parkinson’s Disease
OverviewInbrija is a prescription drug approved by the U.S. Food and Drug Administration to treat off episodes in people with Parkinsons disease who are taking oral levodopa/carbidopa.
Inbrija is an inhaled formulation of levodopa. Levodopa is the precursor molecule to the neurotransmitter dopamine. Lowered levels of dopamine are the cause of motor symptoms in people with Parkinsons. Levodopa is believed to treat Parkinsonian motor symptoms by increasing the concentration of dopamine in the brain.
How do I take it?Inbrija can be taken a maximum of five times a day.
Inbrija comes in the form of powder capsules that are orally inhaled using a special inhaler.
Side effectsThe FDA-approved label for Inbrija lists common side effects including cough, nausea, upper respiratory tract infection, and discolored saliva.
Rare but serious side effects listed for Inbrija include falling asleep during daily activities, impulse control, and psychotic symptoms such as hallucinations.
For more details about this treatment, visit:
Inbrija Acorda Therapeutics
Also Check: On-off Phenomenon
What Are Reasons I Shouldnt Take Inbrija
There are several reasons why Inbrija may not be the right medication for you.
You should not take Inbrija if you:
- Currently are taking a nonselective monoamine oxidase inhibitor like Nardil or Parnate
- Have an underlying chronic lung disease like chronic obstructive pulmonary disease or asthma: Inbrija can cause wheezing or trouble breathing.
- Have a sleep disorder or are taking another medication that makes you drowsy or sleepy
- Have closed angle glaucoma, as it is an absolute contraindication: Talk to your healthcare provider about the risk vs. benefit of taking this medication in this case.
Inbrija In Trials For Parkinsons
Inbrija has been the focus of several clinical trials in Parkinsons disease.
Acorda announced positive results from one of these trials, the Phase 3 SPAN-PD trial , in 2017. The double-blind, randomized trial assessed the safety and effectiveness of Inbrija in treating off episodes compared with a placebo in 351 participants with Parkinsons.
The results demonstrated that patients taking 84 milligrams of Inbrija had significantly improved motor function compared with those in the placebo group.
Acorda has also completed two Phase 3 trials that assessed the long-term safety of Inbrija.
One trial evaluated changes in lung function in 408 Parkinsons patients. Individuals were given either 84 mg of Inbrija up to five times daily together with standard of care, or standard of care alone. The results showed no significant difference between the treatment and control groups, suggesting that Inbrija does not affect lung function.
This trial also found that Inbrija decreased Parkinsons-related symptoms when standard treatment wears off, and it reduced the length of off periods.
Another trial enrolled 325 patients who completed the SPAN-PD trial, with the aim of assessing the long-term safety of both low and high doses of Inbrija for a further year. The safety profile was similar to previous trials. The most common side effects associated with Inbrija were cough, cold, involuntary muscle movements, and falls.
Recommended Reading: Judy Woodruff Parkinson’s
Inhaled Levodopa In Parkinson’s Disease Patients With Off Periods: A Randomized 12
A 12-month, open-label, randomized, controlled study of the pulmonary safety of CVT-301.
-
Patients on standard levodopa regimen were randomized to CVT-301 or observational cohort.
-
Pulmonary safety was assessed using spirometry and carbon monoxide diffusion capacity.
-
There were no notable differences in pulmonary function compared with observational cohort.
-
Exploratory efficacy assessments in the CVT-301 group support clinical efficacy.
How The Technology Works: Inhaled Therapy
The pulmonary route of drug delivery is usually employed to achieve local action of the drug or the needs of systemic drug delivery, primarily by overcoming disadvantages of oral delivery including poor solubility of the drug, oral bioavailability, and the desire to avoid first-pass metabolism .
Nebulizers have been available since the beginning of the twentieth century for pulmonary drug delivery. Most inhaled proteins for clinical development have been developed as liquids for use in nebulizers. Modern inhaler therapy has its origins in treating asthma with pressurized metered-dose inhalers . The commercial dominance of pMDIs was first challenged when chlorofluorocarbon propellants were subject to international control and ultimately phase-out through the Montreal Protocol in 1987 . The difficulty of reformulation of inhaled drugs for alternative propellants and the concurrent demand for alternative delivery systems for the products of biotechnology prompted development and use of dry powder inhalers .
Many drugs are delivered from hydrofluoroalkane propellant-based systems that have recently replaced ozone depleting CFC propellants. The efficiency of delivery is rarely above 50%, despite the slower emission velocity of new propellants. Generally, these inhalers are restricted to low-dose delivery due to physicochemical limitations on drug dispersion in nonpolar propellants and the limited number of successful formulation strategies that are available to overcome this.
You May Like: Weighted Silverware
What Is Inbrija Used For
The Food and Drug Administration approved Inbrija for the intermittent treatment of off episodes in patients with PD taking oral levodopa medication.
Off episodes happen throughout the day when your oral levodopa medicine has either worn off or hasnt taken effect, and your PD symptomslike stiffness, tremor, or anxietyreturn.
Inbrija relieves PD symptoms by changing into dopamine when entering the brain.
Verywell / Zoe Hansen
Long Term Risks Longer Than 3

- Overall, Exablate Neuro is a reasonably safe procedure for treating essential tremor with minimal risk. Infrequent complications that have been reported following Exablate Neuro treatment include long-term numbness and tingling. Additionally, if brain tissue is damaged, there may be muscle weakness, numbness, or sensory loss that may resolve after several months, or it may be non-reversible.
Don’t Miss: Diseases Similar To Parkinsons
Critical Step Forward In Understanding Parkinson’s Disease And How To Treat It
by Dirk Hoffman, University at Buffalo
A new study led by a researcher in the Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo has important implications for developing future treatments for Parkinson’s disease , a progressive nervous system disorder that affects movement and often includes tremors.
“In this study, we find a method to differentiate human induced pluripotent stem cells to A9 dopamine neurons , which are lost in Parkinson’s disease,” says Jian Feng, Ph.D., professor of physiology and biophysics in the Jacobs School and the senior author on the paper published May 24 in Molecular Psychiatry.
“These neurons are pacemakers that continuously fire action potentials regardless of excitatory inputs from other neurons,” he adds. “Their pacemaking property is very important to their function and underlies their vulnerability in Parkinson’s disease.”
“This exciting breakthrough is a critical step forward in efforts to better understand Parkinson’s disease and how to treat it,” says Allison Brashear, MD, UB’s vice president for health sciences and dean of the Jacobs School. “Jian Feng and his team are to be commended for their innovation and resolve.”
Loss of neurons causes Parkinson’s movement symptoms
Feng explains there are many different types of dopamine neurons in the human brain, and each type is responsible for different brain functions.
Quest to develop better treatment therapies
Explore further
People Who Take Salbutamol Are Less Likely To Develop The Condition
- 31 Aug 2017
When people with asthma have trouble breathing, they may reach for an inhaler containing salbutamol, a drug that expands the airways. Salbutamol may have another beneficial effectprotecting against Parkinson’s disease. Individuals who inhaled the highest doses of salbutamol were about half as likely to develop the devastating neurological condition as those who didn’t take the drug, a study reveals.
“I’m sure it’s going to be a landmark paper,” says neurologist Joseph Jankovic of Baylor College of Medicine in Houston, Texas, who wasn’t involved in the research.
In Parkinson’s disease, gobs of the protein -synuclein accumulate in certain brain cells and may kill them. Scientists have tried to craft drugs that speed the elimination of the protein or prevent it from clumping. Neurologist and genomicist Clemens Scherzer of Harvard Medical School in Boston and colleagues decided to try a different strategy. “We wanted to find a drug that could turn down the production of -synuclein,” he says.
How much protection salbutamol provided depended on the dosage. Compared with Norwegians who didn’t use the drug, people who took the highest doses between 2004 and 2007 were about half as likely to get the disease in the subsequent 7 years. In contrast, patients who took the lowest doses had only slightly lower odds of developing Parkinson’s disease in that period, the researchers report online today in Science.
Also Check: Voice Amplifiers For Parkinson’s
Short Term Risks Day Of Treatment Up To 3
- The most common potential risks associated with the Exablate Neuro device and thalamotomy procedure are transient numbness and tingling. These sensations are typically mild to moderate in intensity and can last as briefly as the length of the sonication or up to several days. Headaches or head pain during sonication and imbalance or unsteady were other potential risks, but most often ended shortly after treatment.
- Nausea/Vomiting were also reported in some instances. It is unclear if this is related to medications used during the treatment or the procedure itself.
- You may experience bruising in the area of the iv catheter following the procedure similar to that experienced after blood draws. Any bruising should resolve on its own within a week.
Dosage: How Much Inbrija Should I Take
The dose of this medicine will be different for different patients. Follow your doctor’s orders or the directions on the label. The following information includes only the average doses of this medicine. If your dose is different, do not change it unless your doctor tells you to do so.
The amount of medicine that you take depends on the strength of the medicine. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
- For inhalation dosage form :
- For OFF episodes in patients with Parkinsons disease:
- AdultsTwo 42-milligram capsules taken as needed, for up to 5 times a day. Your doctor may adjust your dose as needed. However the dose is usually not more than 84 mg per OFF episode or 420 mg per day .
- ChildrenUse and dose must be determined by your doctor.
Recommended Reading: On Off Phenomenon
Why Is This Medication Prescribed
Levodopa inhalation is used along with the combination of levodopa and carbidopa to treat ”off” episodes wear off) in people with Parkinson’s disease . Levodopa inhalation will not work to prevent ”off” episodes but will help to control symptoms when an ”off” episode has already begun. Levodopa is in a class of medications called dopamine agonists. Levodopa works by mimicking the action of dopamine, a natural substance in the brain that is lacking in patients with PD.
What Other Medications Interact With Inbrija
Some drugs may interact with Inbrija. If you are taking a medicine that interacts with Inbrija, your healthcare provider may need to choose an alternative medicine and/or monitor you more closely.
For example, certain dopamine antagonists like the antipsychotic drugs Risperdal and Reglan , which stimulate your gut muscles, may make Inbrija less effective in your body.
Similarly, an antibiotic used to prevent and treat tuberculosis, called isoniazid , may also reduce how well Inbrija works. Additionally, iron salts, like those found in some multivitamins, can impair the absorption of Inbrija.
The above list may not be inclusive of all drugs that can interact with Inbrija.
Before starting Inbrija, tell your healthcare provider about all the medications you are taking, including prescription and over-the-counter drugs, herbal products, supplements, vitamins, and recreational drugs.
Recommended Reading: Sam Waterston Parkinson’s