Parkinson’s Diseaseinfusion Pump And Apomorphine Therapy
The disease is named after James Parkinson, a 19th century London surgeon, who was the first to describe its symptoms in a book: âA Treatise on Agitating Paralysisâ. Parkinson’s disease is characterised by the slow but progressive degeneration of the nerve structures that make up the extrapyramidal system.
The first symptoms of Parkinson’s disease emerge when the production of dopamine is considerably reduced, and the main motor disorders are characterised by: tremor at rest, stiffness, slow movement and balance.
Parkinson’s disease generally affects individuals over the age of fifty, the causes of which are not yet fully known.
Currently, there is no cure to completely recover from Parkinson’s disease, but there are treatments to improve the quality of life of those living with this disease. Attack therapy for the treatment of Parkinson’s disease aims to replace dopamine through the administration of levodopa or oral dopamine agonists. While it is true that oral therapy for the treatment of Parkinson’s disease can significantly improve the clinical characteristics for several years, up to 80% of patients develop complications to the motor response, characterised by motor fluctuations such as an end-of-dose effect and dyskinesias.
Subcutaneous administration of apomorphine, using a portable infusion pump, has been shown to be effective in controlling the complications of the disease.
What Are The Ingredients In Apomorphine
Active ingredient: apomorphine hydrochloride, USP
Inactive ingredients:
Apomorphine injection : sodium metabisulfite, NF, benzyl alcohol, NF, water for injection, USP. It may also contain sodium hydroxide, NF and/or hydrochloric acid, NF.
Apomorphine sublingual film : disodium EDTA dihydrate, FD& C Blue #1, glycerol, glyceryl monostearate, hydroxyethyl cellulose, hydroxypropyl cellulose, maltodextrin, -menthol, pyridoxine hydrochloride, sodium hydroxide, sodium metabisulfite, sucralose, and white ink.
Apomorphine injection is distributed under the brand name Apokyn by MDD US Operations, LLC, Rockville, MD 20850. TruPharmac, LLC also produces a generic version of apomorphine injection
Apomorphine sublingual film is manufactured under the brand name Kynmobi for Sunovion Pharmaceuticals Inc. Marlborough, Massachusetts 01752 USA.
Alternative Apomorphine Delivery Strategies
To date, the main administration route for apomorphine in PD has been subcutaneous, either as a continuous infusion or as an intermittent pen injection. This route has proven effective, but skin reactions are among the most common adverse events and can complicate treatment or lead to withdrawal. For some patients, this delivery may also be problematic because of needle phobia for others, the pen injection may prove challenging for resolving an acute off phase because of bradykinesia and tremor. Despite its remarkable efficacy, apomorphine suffers from the lack of an easier and less invasive delivery system. Several alternative delivery routes have therefore been tested, and some are in active clinical development.
Oral apomorphine is considered infeasible because of the almost complete first-pass hepatic metabolism of the molecule . However, the administration of apomorphine and its prodrug via oral lipid-based formulations has recently been reported in animal models of PD. This formulation is still in the preclinical phase but may have the potential to achieve steady dopaminergic stimulation because of its sustained drug release .
Don’t Miss: Parkinson Bicycle Cleveland Clinic
Falling Asleep During Activities Of Daily Living And Somnolence
There have been reports in the literature of patients treated with apomorphine hydrocloride subcutaneous injections who suddenly fell asleep without prior warning of sleepiness while engaged in activities of daily living. Somnolence is commonly associated with apomorphine hydrocloride, and it is reported that falling asleep while engaged in activities of daily living always occurs in a setting of pre-existing somnolence, even if patients do not give such a history. Somnolence was reported in 35% of patients treated with apomorphine hydrocloride and in none of the patients in the placebo group. Prescribers should reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
How Should I Use This Medicine

This medicine is for injection under the skin. You will be taught how to prepare and give this medicine. You will also need to take a medicine prescribed by your doctor to prevent nausea and vomiting when you first start treatment. Use exactly as directed. Do not take your medicine more often than directed. Do not stop taking except on your doctor’s advice. Stopping this medicine too quickly may cause serious side effects.
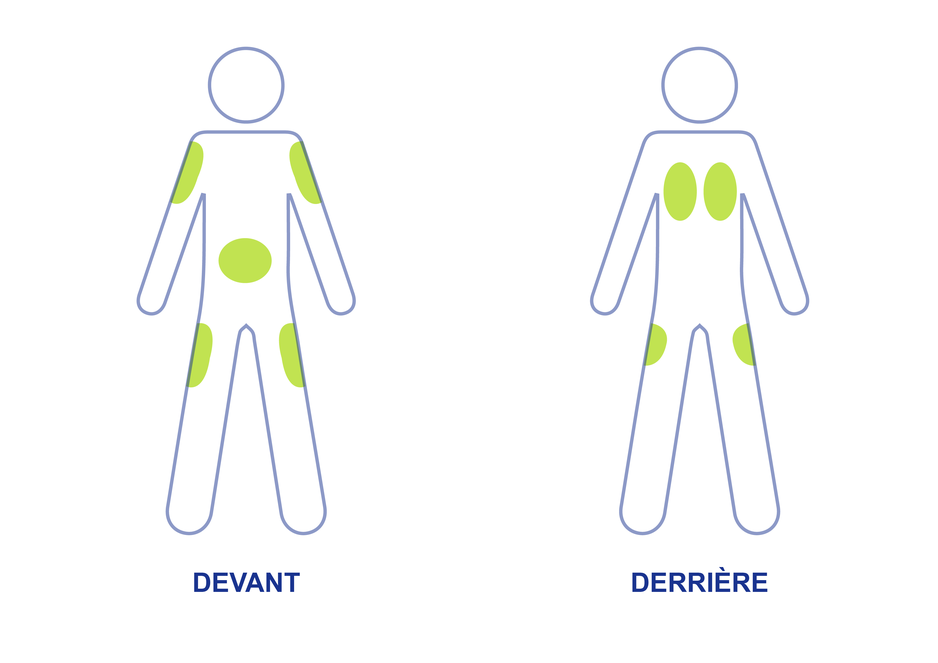
It is important that you put your used needles and syringes in a special sharps container. Do not put them in a trash can. If you do not have a sharps container, call your pharmacist or healthcare provider to get one.
Talk to your pediatrician regarding the use of this medicine in children. Special care may be needed.
Overdosage: If you think you have taken too much of this medicine contact a poison control center or emergency room at once.
NOTE: This medicine is only for you. Do not share this medicine with others.
Recommended Reading: Parkinson’s Bike Therapy
Early Clinical Data In Pd
The early work of use of apomorphine in PD patients was conducted mainly by Schwab et al. in the 1950s. While these early trials showed apomorphines efficacy for treatment of Parkinson symptoms, patients reported marked side effects and required high oral doses in order to see an effect. The drug languished until 1970, when fast acting, injectable subcutaneous preparations were developed . Patients with PD had a similar response in motor symptoms, including tremor, with apomorphine as compared to l-Dopa . That same year, Braham et al. showed improvement in tremor within 510 min lasting 12 h following subcutaneous injections of 0.52 mg apomorphine in 11 of 15 Parkinson patients with l-Dopa resistant tremor. Side effects in the early trials consisted of syncope and vomiting, and were frequent .
What Is Apomorphine Used For
Apomorphine injection is a prescription medicine used to treat acute, intermittent episodes of poor mobility called ” off ” episodes in people with advanced Parkinson’s disease.
Apomorphine sublingual film is a prescription medicine used to treat short-term , intermittent off episodes in people with Parkinson’s disease.
It is not known if apomorphine is safe and effective in children.
You May Like: Voice Amplifiers For Parkinson’s
Sublingual And Nasal Administration
Due to the chemical nature of apomorphine and its highly oxidative property, finding a means to stabilize apomorphine for delivery is essential. The oxidation of the molecule is increased with an alkaline environment, light and oxygen. This has constrained routes of delivery, due to apomorphines rapid degeneration in solutions intended for either oral or nasal administration. The highly acidic properties of apomorphine also contribute to stoma formation in oral/sublingual administrations, as well as severe nasal lining irritation. Research has been done investigating different systems that utilize stabilizing buffers integrated with the active solid apomorphine in an effort to reduce the acidity .
A new insufflation system that utilizes the method of time-of-administration mixing to activate a less irritating, but also less stable, form of apomorphine is currently under clinical investigation . Early results and observances have found this novel nasal delivery has similarly rapid onset of action, while minimizing adverse events, specifically nasal irritation. In addition to nasal delivery systems, new pulmonary delivery systems are also being investigated. Pulmonary inhalation would allow for larger surface area leading to rapid absorption .
Dosage Forms And Strengths
Apomorphine hydrocloride 30 mg/3 mL containing apomorphine hydrochloride , USP is supplied as a clear, colorless, sterile, solution in a 3 mL cartridge. The 3 mL glass cartridge for single-patient-use is used with a manual reusable pen injector which is supplied separately by a different manufacturer. A single cartridge, pen and needle can deliver doses up to 1 mL in 0.02 mL increments. The APOKYN® pen injector is provided in a package with six needles.
Also Check: Does Sam Waterston Have Parkinsons
What Are The Its Disadvantages
Efficacy Of Apomorphine For Non
Non-motor symptoms of PD are now known to occur throughout all stages of the disease and negatively impact the quality of life of these patients. NMS include neuropsychiatric and gastrointestinal symptoms, sleep disturbances , urinary dysfunction, pain and impulse control disorders. While no formal studies have been conducted looking at the direct effect of apomorphine on NMS, there is enough evidence from the numerous trials focused on motor symptoms of PD to suggest a beneficial effect of continuous infusion of apomorphine on several NMS in patients with PD . Focused studies need to be explored in the future.
You May Like: Judy Woodruff Health Problems
What Side Effects May I Notice From Receiving This Medicine
Side effects that you should report to your doctor or health care professional as soon as possible:
- allergic reactions like skin rash, itching or hives, swelling of the face, lips, or tongue
- breathing problems
- falling asleep during normal activities like driving
- feeling faint or lightheaded, falls
- hallucination, loss of contact with reality
- increased sweating
- males: prolonged or painful erection
- signs and symptoms of a dangerous change in heartbeat or heart rhythm like chest pain dizziness fast or irregular heartbeat palpitations feeling faint or lightheaded, falls breathing problems
- signs and symptoms of low blood pressure like dizziness feeling faint or lightheaded, falls unusually weak or tired
- swelling in arms, hands, legs, or feet
- uncontrollable and excessive urges
- uncontrollable head, mouth, neck, arm, or leg movements
- vomiting
Side effects that usually do not require medical attention
- drowsiness
- pain, redness, or irritation at site where injected
- runny nose
- yawning
This list may not describe all possible side effects. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Side Effects Of Apomorphine Usage In Parkinsons Disease
Stuart Isaacson, MD: Like any therapy, there are advantages and disadvantages. We know orthostatic hypotension is about 11%, and nausea and vomiting. Bill, youve looked specifically at the use of the apomorphine injection subcutaneously in terms of nausea and the use of Tigan thats recommended on the label. What are your feelings on this because weve have some difficulty in the United States of having availability of Tigan now?
William G. Ondo, MD: Nausea is one of the main potential adverse effects of any apomorphine preparation. In the studies, Tigan has been used, which is kind of a dirty drug but largely an antihistamine-type medication. The first person who did one of the studies in the United States in the early 90s used Tigan, and it became dogma that you use Tigan for this.
We looked at a real-world open-label series in patients who took Tigan versus patients who did not, and we really didnt see any increase in nausea in patients who were not taking Tigan. In fact, there was a trend toward increased nausea in patients who took Tigan. That was not a controlled trial in any way, shape, or form. I cant speak for other antinausea medications, most of which you cant use in Parkinson disease because they block dopamine receptors, which makes them contraindicated. But there isnt compelling information that these medicines reduce the rates of nausea with apomorphine.
Transcript edited for clarity.
Read Also: Fitflop Shoes For Parkinson’s
Compliance With Ethical Standards
No sources of funding were used to conduct this study or prepare this manuscript. Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Federico Carbone has no conflicts of interest that are directly relevant to the content of this review. Atbin Djamshidian has received consulting fees from Abbvie and Grünenthal. Klaus Seppi has received fees from Teva, UCB, Lundbeck, AOP Orphan Pharmaceutical AG, Roche, Grünenthal and Abbvie honoraria and research grants from the International Parkinson and Movement Disorder Society and research grants from the FWF Austrian Science Fund and Micheal J. Fox Foundation outside of the submitted work. Werner Poewe has received consulting fees from Britannia Pharmaceuticals for planning and implementation of the TOLEDO study consulting fees from Grünenthal in relation to apomorphine educational activities and lecture fees from Britannia Pharmaceuticals and Grünenthal related to symposia on the TOLEDO study and for apomorphine educational activities.
Antihypertensive Medications And Vasodilators
In clinical studies, the following adverse events were experienced more commonly in patients receiving concomitant antihypertensive medications or vasodilators than in patients not receiving these medications : hypotension , myocardial infarction , serious pneumonia , serious falls , and bone and joint injuries . Some of the events may be related to the increased incidence of hypotension in patients receiving concomitant antihypertensive medications or vasodilators .
Concomitant administration of 0.4 mg sublingual nitroglycerin with apomorphine hydrocloride in healthy subjects causes greater decreases in blood pressure compared to apomorphine hydrocloride alone. When nitroglycerin and apomorphine hydrocloride were concomitantly administered to healthy subjects, the mean largest decrease in supine systolic and diastolic blood pressure was 9.7 mm Hg and 9.3 mm Hg, respectively . The mean largest decrease in standing systolic and diastolic blood pressure was 14.3 mm Hg and 13.5 mm Hg, respectively. Some individuals experienced very large decreases in standing systolic and diastolic blood pressure, up to a maximum decrease of 65 mm Hg and 43 mm Hg, respectively.
In comparison, the mean largest decrease in supine systolic and diastolic blood pressure when apomorphine hydrocloride was administered alone was 6.1 mm Hg and 7.3 mm Hg, respectively, and in standing systolic and diastolic blood pressure was 6.7 mm Hg and 8.4 mm Hg, respectively.
Read Also: Cleveland Clinic Parkinson’s Bicycle Study 2017
Premedication And Concomitant Medication
Because of the high incidence of nausea and vomiting with apomorphine hydrocloride treatment, an antiemetic, e.g., trimethobenzamide 300 mg three times a day, should be started 3 days prior to the initial dose of apomorphine hydrocloride . Treatment with trimethobenzamide should only be continued as long as necessary to control nausea and vomiting, and generally no longer than two months after initiation of treatment with apomorphine hydrocloride, as trimethobenzamide increases the incidence of somnolence, dizziness and falls in patients treated with apomorphine hydrocloride .
Based on reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron, the concomitant use of apomorphine with drugs of the 5HT3 antagonist class including antiemetics and alosetron are contraindicated .
Can Apomorphine Cause Problems
Along with their useful effects, most medicines can cause unwanted side-effects although not everyone experiences them. The table below contains some of the most common ones associated with apomorphine. You will find a full list in the manufacturer’s information leaflet supplied with your medicine. The unwanted effects often improve as your body adjusts to the new medicine, but speak with your doctor or pharmacist if any of the following continue or become troublesome.
| Common apomorphine side-effects | |
| Stand up slowly and get your balance before you start to walk | |
| Yawning, confusion, imagining things that are not real | If any of these become troublesome, speak with your doctor |
Important: speak with your doctor as soon as possible if you notice any of the following:
- Changes in your behaviour, such as a desire to gamble or an increased sex drive.
- Falling asleep suddenly.
Don’t Miss: Zhichan Capsule
Apomorphine In Parkinsons Disease: Practical Considerations
CSAI is administered via a portable pump system that delivers a continuous dose, with the possibility of releasing a rescue bolus if needed. The duration of infusion is normally 1216 h , but a 24-h regimen can also be programmed for patients experiencing nocturnal hypokinesia . Patients with PD who have off periods no longer controlled with optimized oral therapy or who need apomorphine rescue doses too frequently are suitable candidates for CSAI. The pump can also be an alternative to surgical therapy or to enteral levodopa infusion . For patients starting CSAI in the inpatient setting, domperidone 10 mg three times daily from 1 day before initiation to 37 days in total is strongly recommended to prevent nausea . On the first day, apomorphine treatment is started at a dose of 0.5 or 1 mg/h. Uptitration is usually with 0.5 or 1 mg/h daily increments, and the optimal infusion rate ranges from 4 to 7 mg/h for most patients. Concomitantly, oral dopamine agonists and other antiparkinsonian drugs are gradually discontinued. During the titration phase, levodopa is also usually reduced, and discontinued if possible in patients with dyskinesia . The same uptitration protocol should be used for outpatients but with a slower increase in infusion rates.
What May Interact With This Medicine
Do not take this medicine with any of the following medications:
- cisapride
- nitroglycerin
- other medicines that prolong the QT interval
This list may not describe all possible interactions. Give your health care provider a list of all the medicines, herbs, non-prescription drugs, or dietary supplements you use. Also tell them if you smoke, drink alcohol, or use illegal drugs. Some items may interact with your medicine.
Recommended Reading: Prayer For Parkinson’s Disease
Effect On Off Periods
The patients motor status was recorded by a specialised nurse every 30 minutes during the awake part of the day for at least 2 days immediately before the start of treatment, during the entire dose finding and education period, and for 48 hours at every evaluation visit. The patients also kept daily on-off diaries for at least 1 week before each follow up visit.
Current And Future Delivery Methods: Tolerability

The most common current preparations are subcutaneous injections to abort off periods in patients taking l-Dopa and continuous subcutaneous infusions, which are administered throughout the day. Both have been used for many years but continue to be refined . Sublingual and nasal preparations have not been successfully developed to this point but new preparations are being tested . Specific preparations have unique side effects but the drug itself has several consistent problems. The most common issues are nausea and vomiting, and hypotension including syncope. These tend to occur most dramatically with dose initiation, and improve with continued use, but often still result in discontinuation. Patients are typically pretreated with an anti-emetic in an effort to decrease nausea. In the past, the majority of trials pretreatment was with domperidone, but more recent US trials used trimethobenzamide as pretreatment because domperidone is not available . Patients often yawn just prior to clinical improvement. This is an interesting, though not usually problematic side effect . Other side effects typical for DAs are also seen: sedation, hallucinations, and impulse control disorders.
Recommended Reading: On Off Phenomenon