Practical Issues Selection Of The Surgical Target Technique And Programing
Table 1 shows a summary of points that need to be considered when indicating these DBS techniques.
Numerous studies have demonstrated the effectiveness of STN DBS in controlling the appendicular motor signs of PD, however, this procedure is not considered to have as much of a direct effect on the intensity of LID. The anti-dyskinetic effect of STN DBS have been hypothesized to be related to allowing reduction of dopaminergic drug dosages, with consequent improvement in side effects, including LID. The persistence or worsening of LID after STN DBS is common and is, in fact, indicative of the necessity to reduce the dose of levodopa . Therefore, the si ne qua non-condition for reduction of LID when STN DBS is considered, is its capacity to enable a reduction of levodopa dosage. If, however, an adequate response of motor symptoms does not occur post-operatively, dyskinesia will remain unchanged. Of importance, STN stimulation not uncommonly induces contralateral dyskinesia, which may be persistent, and in some cases lead to the implantation of rescue GPi leads.
In the case of a patient in whom, in addition to motor signs of parkinsonism, medication side effects other than dyskinesia are a primary source of disability , STN DBS may be more desirable.
Post-operative programing: GPi DBS
Post-operative programing: STN DBS
What Causes Dyskinesia In Parkinsons Disease
In Parkinsons disease, there is a significant loss of dopaminergic nerve cells in the brain. Dopaminergic neurons control voluntary movements and behavioral processes such as mood, reward, addiction, and stress. The loss of dopaminergic neurons affects regular body movements and results in symptoms such as involuntary shaking of hands, arms, legs, jaw, and the tongue, slowness of body movement , stiffness in the arms and legs, and gait and balance problems.
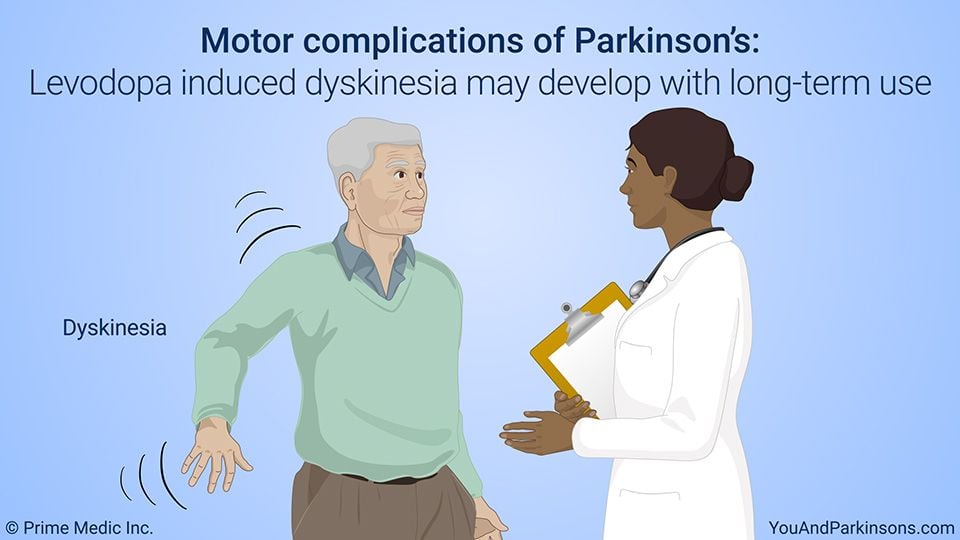
Levodopa is one of the most potent medications available for Parkinsons disease. It is a precursor of dopamine, or a molecule that is converted to dopamine in the body. Unlike dopamine, levodopa can cross the blood-brain barrier and replenish dopamine levels in the brain, relieving symptoms of Parkinsons disease. However, because levodopa is taken intermittently during the day, its levels in the blood fluctuate. Dyskinesia usually begins after two to three years of levodopa treatment and becomes progressively worse. Nearly half of levodopa-treated patients display some form of dyskinesia after levodopa treatment for five years.
There are two kinds of dyskinesia: peak dyskinesia and diphasic dyskinesia. Peak dyskinesia occurs when the concentration of levodopa in the body is at a maximum. Diphasic dyskinesia occurs when the levels of levodopa are rising or falling. This type of dyskinesia is generally associated with low-dose levodopa treatment.
D1r And Darpp Intracellular Signaling After L
Considering that LID is associated with modifications in the D1R-containing neurons, studies indicate that L-DOPA increases PKA activation, and DARPP-32 phosphorylation only in the medium-sized spiny neurons of the direct pathway . Under basal conditions the phosphorylation of DARPP-32 is low at the Thr34 and high at the Thr75, Ser97, and Ser130 residues . In dopamine-denervated animals, the hypersensitization of D1R after L-DOPA is reflected in robust phosphorylation of DARPP-32 at Thr34 and dephosphorylation at Thr75 and Ser97, leading to changes in downstream signaling cascades and transcriptional activation of many genes like Arc, c-Fos, FosB, zif-268, and brain-derived neurotrophic factor in direct pathway neurons . In agreement, dyskinesia is attenuated in DARPP-32 global KO mice and in mice lacking DARPP-32 selectively in the MSN of the direct pathway, but not in those of the indirect pathway . These studies indicate the importance of enhanced cAMP/PKA/DARPP-32 signaling in LID, and point to the MSN of the direct pathway as a key neuronal substrate for LID.
Figure 1et al
Recommended Reading: New Parkinson’s Medication For Hallucinations
When Do You Get Dyskinesia
Most people are on levodopa for 5 to 10 years before they notice dyskinesia. And it usually starts when Parkinson’s is under good control. This is called peak dyskinesia because it happens when your dopamine levels are highest. After a while, symptoms may start sooner and last longer than this peak time.
But they still happen when levodopa is keeping your symptoms in check. Your doctor may call this being âonâ with dyskinesia.
Dyskinesia is sometimes lumped together with a problem called motor fluctuations. But theyâre not the same thing. Motor fluctuations are when Parkinson’s symptoms come back during times your meds arenât working. This can happen if levodopa wears off before you take your next dose or a new dose doesnât kick in right away.
What Are Dyskinesias And How Can I Manage Them

Dyskinesias are abnormal, involuntary movements that occur in response to repeated dopamine-replacement therapy . Sometimes, they can be debilitating. These motor complications are typically choreiform. Chorea comes from the Greek word meaning to dance, so the dyskinesias looks similar to dance-like, constant writhing or wriggling movements of the arms, legs, trunk, and sometimes even facial muscles. However, dyskinesias can also be dystonic , or myoclonic or other movement disorders, and can become progressively more severe with increasing duration of treatment . Sometimes, with advancing disease, it becomes increasingly difficult to find a dose of levodopa that provides symptom relief while avoiding dyskinesia.
You May Like: Tai Chi And Parkinson’s
Pharmacologic Strategies To Directly Address The Incidence Of Dyskinesias
Restoration of striatal dopaminergic stimulation is the goal in the treatment of parkinsonian motor symptoms. Levodopa provides the greatest benefit for treating parkinsonian motor dysfunction, but because its use is associated with the development of motor complications, one of the great unmet needs for the treatment of PD is a medication that will match the efficacy of levodopa but not cause motor complications. Until such a medication is available, it is useful to identify treatment strategies that can provide adequate efficacy while minimizing motor complications.
The short half-life of levodopa and the resultant pulsatile dopaminergic stimulation appear at least in part to be responsible for the development of motor complications . Therefore, CDS may delay the onset of dyskinesias in early disease and alleviate dyskinesias in advanced disease.
Mechanism Mediating Tms Efficacy
TMS uses electromagnetic induction by means of a rapidly changing magnetic field to induce weak electric currents that provoke activity in specific or diffuse parts of the brain, allowing the brain functioning and interconnections to be studied. rTMS could be a therapeutic tool in movement disorders, in particular by creating long-lasting changes in synaptic excitability within the motor system as a way to modulate symptoms. The motor improvement after rTMS could be attributed to its effects on the motor cortex or the supplementary motor area , or to its ability to induce dopamine release from the basal ganglia . The effects of rTMS and its functional outcome in PD patients are still unclear with no consensus on the symptoms most likely to respond. Therefore it can be perceived that the study of TMS as a treatment in PD and in LID needs a large multicenter trial taking into account the interindividual variability observed in PD patients, and considering the profile of cortical plasticity and its modulation by dopamine .
Recommended Reading: Fluid Retention And Parkinson’s
How Dbs Helps Dyskinesia
The mechanism by which DBS helps reduce dyskinesia is fairly involved. The device induces brain stimulation, which can excite or suppress brain activity. Depending on the location of the electrodes, the electrical stimulation may reduce dyskinesia by direct action on the brain, or it may indirectly reduce dyskinesia by reducing the need for dopaminergic medication, which in turn, reduces the dopaminergic side effect of dyskinesia.
Stimulators placed in the globus pallidus directly impact the dyskinesias, while stimulators placed in the subthalamic nucleus can reduce the need for dopaminergic medication, diminishing the side effect of dyskinesia.
How Do I Know If Its Parkinsons Disease Dyskinesia Or A Parkinsons Tremor
At times, it can be difficult to distinguish between Parkinsons Disease Dyskinesia and parkinsonian tremor, particularly when the information is based primarily on history. Making the correct diagnosis is critically important, as it can profoundly alter treatment decisions. If a person with Parkinsons is having dyskinesias that are bothersome and/or present most of the time, one option would be to reduce the levodopa dose. On the other hand, if a person with Parkinsons is experiencing a parkinsonian tremor, one would do the exact opposite .
In short, making the wrong decision such as increasing levodopa when the person with Parkinsons actually has bothersome dyskinesias or decreasing the dose when the person with Parkinsons actually has tremor, can significantly compromise normal movement and quality of life.
First and foremost, your doctor must take the time to educate him/herself on your condition before making this distinction. As noted above, dyskinesias are highly irregular and cause a variety of types of movements. Parkinsonian tremor is quite different. It can affect one or more parts of the body and is characterized by regular back and forth oscillations of movement with a frequency of 5-7 cycles per second. If recorded, one would see rhythmic sinusoidal waves of to and fro movements.
Also Check: Is There Pain With Parkinson’s
Clinical Features And Classification Of Lid
LID are clinically heterogeneous. They commonly present as chorea or choreoathetosis, though myoclonus, akathasia, ballism and other forms of abnormal movements have also been described. LID generally first appear on the side worst affected by Parkinson’s disease and in legs before arms. This could be related to an early dopaminergic loss in the dorsolateral striatum, the region corresponding somatotopically to the foot area.
Chorea refers to involuntary, rapid, irregular, purposeless, and unsustained movements that seem to flow from one body part to another. The severity of these movements can vary from occasional abnormal movements that are absent at rest and provoked only during active movementfor example, walking or talking to violent large amplitude flinging and flailing arm movementsthe ballism. Often, there are superimposed writhing athetoid movementschoreoathetosis. Dyskinesias may predominantly affect particular body partsfor example, torso, head and neck, limbsor speech or respiratory muscles.
Dystonia is the second most common form of LID presenting as sustained muscle contractions. It occurs either in pure form or in combination with the chorea, in the latter case manifesting as twisting of the leg on walking or the arm being pulled behind the back. Dystonia accounts for greater disability than chorea. Off time dystonias are usually painful.
Carbidopa And Levodopa Extended Release Capsules
Carbidopa and levodopa extended release is an oral formulation of levodopa designed to combine both immediate release and extended release pharmacokinetics, allowing for less frequent dosing and more stable and longer lasting plasma concentrations of levodopa compared to other formulations of oral levodopa. CD-LD ER capsules contain 4 varieties of beads: one with immediate release carbidopa-levodopa, two with different extended release carbidopa-levodopa formulations, and a fourth with an active excipient containing tartaric acid to facilitate enteral absorption .
Pharmacokinetic studies have demonstrated that CD-LD ER provides a rapid rise in plasma levodopa concentration with prolonged duration relative to other oral levodopa formulations . In an open-label, randomized crossover study of CD-LD ER and CD-LD IR, the time to Cmax was similar for both drugs , but the duration of levodopa concentration above 50% of Cmax was 2.6 h longer for CD-LD ER versus CD-LD IR. Following a single dose, improvements in UPDRS part III scores were similar for both medications up to 2 h post-dosing, but for hours 3 through 6 UPDRS part III scores were significantly more improved with CD-LD ER than CD-LD IR. According to clinicians ratings, at 6 h, 68% of subjects were rated as ON without troublesome dyskinesia after taking CD-LD ER compared to 4% after taking CD-LD IR .
Read Also: All About Parkinson’s Disease
Drugs Acting On Serotonergic Systems
The basal ganglia have dense serotonergic innervation. It is suggested that serotonergic transmission has an inhibitory effect on dopaminergic transmission. There are reports of successful use of 5HT agents in treating LIDs., However, these studies included very small numbers and were mostly uncontrolled.
Safinamide And Its Competitors
Safinamide combines two well-known and proven pharmacologic principles in the treatment of PD: inhibition of MAO-B and decline of abnormal glutamate release. One may postulate that safinamide resembles rasagiline or selegiline. However, safinamide does not act in an irreversible fashion on MAO-B enzyme activity like rasagiline or selegiline. Therefore, it is not just another MAO-B inhibitor. Safinamide does not block NMDA receptor function. NMDA receptor antagonism is the mode of action of amantadine. This agent has also some anticholinergic features in addition to its dopamine-mimicking properties. In contrast, safinamide modulates sodium- and potassium-ion channels in a way that finally induces a declined abnormal glutamate release. Thus, safinamide is different from NMDA antagonists.,
Also Check: Non Shaking Parkinson’s Disease
Pharmacokinetic And Metabolic Properties Of Safinamide
Pharmacokinetic behavior
The gastrointestinal absorption of safinamide is good and fast. The maximum concentration was observed within 24 hours. The absolute bioavailability was 95%. Steady-state concentrations were reached within 1 week. Plasma protein binding was within a range between 88% and 90% with a volume distribution of ~165 L, which corresponds to 2.5-fold of the body volume. There was an extensive extravascular distribution of safinamide. The terminal half-life was about 22 hours . The total clearance was 4.6 L/hour .
Metabolic characteristics
Extensive degradation of safinamide is predominantly undertaken via amide hydrolytic oxidation with safinamide acid generation as main derivative . There are further less important metabolic pathways. Ether bond oxidation synthesizes an O-debenzylated safinamide. Oxidative turnover of safinamide or safinamide acid generates the N-dealkylated acid. All these derivatives possess no pharmacologic activity. The beta-glucuronide of the N-dealkylated acid and the monohydroxy safinamide appeared in urine., The glycine conjugate of the N-dealkylated acid and 2- propanamide are minor urine derivatives of safinamide., Mild-to-moderate impairment of hepatic function may rise safinamide levels within a range between 30% and 80%.
Acknowledgements And Conflict Of Interest Disclosure
RM acknowledges grants from the Spanish Ministries de Economía y Competitividad and of Sanidad Política Social e Igualdad, ISCIII: BFU2010-20664, PNSD, CIBERNED ref. CB06/05/0055 and Comunidad de Madrid ref. S2011/BMD-2336, JRGM is supported by ICyTDF México MTH acknowledges the support by CIBERNED CB05/05/505, SAF2007-062262 and FIS PI10-02827. RH and KC were supported by the German Bundesministerium für Bildung und Forschung, Grant 01GN1006B. NS gratefully acknowledges Sardinia Regional Government for financial support . The authors have no conflicts of interest to declare.
All experiments were conducted in compliance with the ARRIVE guidelines.
Recommended Reading: Is Beer Good For Parkinson’s
Transcranial Magnetic Field Stimulation
TMS is a safe and non-invasive method that affects the cerebral cortex but not the deep structures. TMS can be used to investigate causality in the brain-behavior relationship by utilizing neuronal depolarization or hyperpolarization .
Two primary protocols are established for TMS. In the first one, the single/paired-pulse TMS depolarizes and discharges the action potential under the region that is being stimulated. When applied to the motor cortex, s/p-pTMS can provoke motor evoked potentials, an output commonly used together with TMS . The second one, involves the repetitive TMS which has been shown to produce more durable effect after the initial stimulation . rTMS can increase or decrease the excitability of the corticospinal tract depending on the intensity of stimulation, coil orientation and frequency, and properties of the stimulation coil. The inhibitory/excitatory effects of TMS on the neurons should be distinguished from the negative/positive outcome on behavior, which can occur in any combination. For instance, an excitatory effect of TMS in one area may induce inhibition of a different area that controls the execution of cognitive tasks, resulting in a negative behavioral outcome .
Whats The Cause Of Parkinsons Disease Dyskinesia
Parkinsons Disease Dyskinesia is generally believed to be caused by disease progression and the use of levodopa medications. As Parkinsons progresses, deteriorating dopamine brain cells have increasing difficulty in managing normal movement. Levodopa medications replace normal dopamine allowing good movement control which is less OFF time and more ON time .
Over time it may be necessary to take more frequent doses of levodopa to manage movement problems. When levodopa is administered in frequent doses , the levels in the blood vary between doses, creating peak and troughs rather than continuously replacing dopamine. This has the potential to damage dopamine receptors over the years, eventually causing Parkinsons Disease Dyskinesia.
You May Like: What Causes Tremors Besides Parkinson’s
Why Dyskinesia Develops As A Result Of Parkinsons Disease Medications
Parkinsons disease is a movement disorder identified by resting tremors and muscle rigidity. Medications used for Parkinsons disease are among the most recognized causes of dyskinesia. The medications that are used to control the symptoms of Parkinsons disease are called dopaminergic medications. As these dopaminergic medications increase the amount of dopamine in the brain, they effectively reduce the symptoms of Parkinsons disease.
Dyskinesia does not typically occur as the result of only a few doses of dopaminergic medications or when using these medications for a short period of time. Because Parkinsons disease is a lifelong condition, people who have the disease need to take dopaminergic medications for years. After several years of taking these medications, people with Parkinsons disease may develop a fairly common delayed side effect of dyskinesia.
There has been a great deal of investigation into whether it is possible to prevent the dyskinesias from developing and whether postponing dopaminergic medications can delay or reduce the development or severity of dyskinesia. But there has not been convincing evidence to show that delaying dopaminergic medication can prevent this side effect from eventually developing or make it less severe in the long run.
Involvement Of Erk In Dyskinesia
Molecular changes related to LID have been detected in other signaling cascades associated with dopamine D1R activation, including the ERK1/2 cascade. D1R stimulation induces ERK1/2 phosphorylation in the dopamine-intact striatum and is strongly potentiated in the dopamine-depleted one . More recent work established that ERK1/2 phosphorylation correlates with increased FosB and dyskinesia, and that the expression pattern overlaps that of FosB in completely denervated striatal areas . This work was substantiated by data demonstrating that the intensity of the p-ERK1/2 signal correlated with the intensity of dyskinesia in mice , and that lovastatin, an inhibitor of Ras/ERK, reduced ERK activity as well as the incidence and severity of LID . Inhibitors of ERK also prevented phosphorylation of mammalian target of rapamycin, and the mammalian target of rapamycin inhibitor rapamycin reduced the development of LID . Interestingly, L-DOPA-induced ERK phosphorylation is more prominent after acute treatment and induces p-ERK preferentially in direct pathway MSN, whereas chronic administration leads to an increase of p-ERK in cholinergic interneurons .
You May Like: Pre Parkinson’s Disease Symptoms