Biopharma Is Tackling Misfolding Proteins In Parkinsons Disease
A progressive neurological condition that causes tremors and mobility problems, Parkinsons disease currently affects nearly one million people in the United States and more than 10 million worldwide, according to the Parkinsons Foundation.
This field is abundant with experimental therapeutics, including one being developed by Gain Therapeutics, which presented at the conference. Gain announced that its lead compound, GT-02287, reduced levels of alpha-synuclein accumulation and neuroinflammation, and decreased behavioral deficits in animals modeling Parkinsons.
We are working diligently to advance this breakthrough compound to the clinic and bring it one step closer to meeting the currently unaddressed needs of patients with this debilitating disease, said Gain CEO Eric Richman in a statement.
Gain has five pipeline products, all of which are in early stages of development or pre-clinical testing. This biotechnology company specializes in enzymes that treat rare genetic diseases and is focused on identifying and optimizing allosteric binding sites never-before targeted in neurodegenerative diseases.
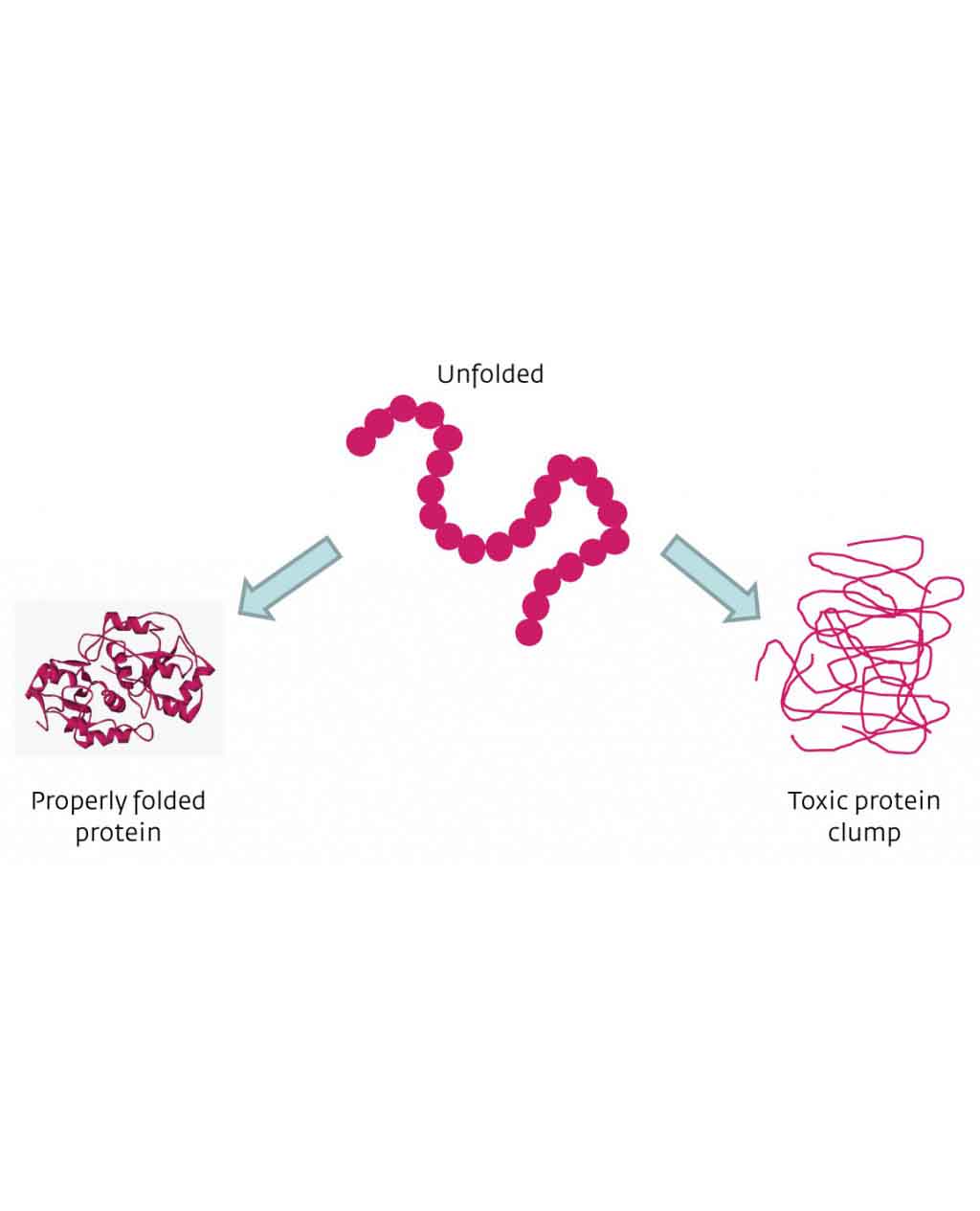
Enzymatic protein misfolding is a characteristic of PD and many other neurodegenerative illnesses. Parkinsons is one of a group of maladies known as ‘protein misfolding diseases’ because they are characterized by specific proteins becoming distorted and malfunctioning. A thread-like chain of these proteins then forms, which is toxic to other cells.
Attacking Mutations
Targeting Production Misfolding And Aggregation
The development of drugs targeting protein misfolding or aggregation has been challenging due to the lack of certainty about which form/s of a given disease protein is primarily responsible for the disease. In the case of amyloid- , it was originally thought that fibrils and plaques were the pathogenic species in Alzheimers disease, but more recent studies point to aggregation intermediates as the primary culprits similar findings have emerged with respect to different species of -synuclein in Parkinsons disease . The situation is further complicated by the existence of the variety of intermediate species that exist during the folding and oligomerization processes. Recent studies have demonstrated that aggregated fibrils of tau, -synuclein, and A exist in different conformational variants, or strains, that have different propagation properties and different levels of neurotoxicity .
Unique Protein Structures Could Hold The Key To Treatment For Parkinsons Disease
Bath scientists have discovered a series of protein structures that are thought to be highly relevant to the onset of Parkinsonâs disease.
- Press release
- View more announcements in Communications
Scientists at the University Bath have discovered a series of protein structures that are thought to be highly relevant to the onset of Parkinsonâs disease. It is hoped that further analysis of these structures will open up a new avenue for potential treatment for a disease that is the fastest growing neurological condition in the world, with no cure currently available.
According to Parkinsonâs UK, more than 1 million people in the UK are affected by the disease – either by living with the condition, or as a friend, colleague, or family member of someone who is. Latest estimates show that in 2020, around 145,000 people live with a Parkinsonâs diagnosis in the UK.
The disease is characterised by a specific protein âmisfoldingâ, where it becomes distorted and then malfunctions. The protein which researchers at Bath have studied â alpha-synuclein â is abundant in all human brains. After misfolding, it accumulates in large masses, known as Lewy bodies. These masses consist of slender αS fibres that are toxic to dopamine-producing brain cells, causing them to die. It is this drop in dopamine that triggers the symptoms of Parkinsonâs Disease.
This study was funded by a BRACE PhD studentship , and both a project grant and equipment grant from Alzheimerâs Research UK .
Recommended Reading: On And Off Phenomenon
The Secretory Compartments In Ups Pathways
On the other hand, the mechanisms by which distinct cargos are targeted to the secretory compartment in different forms of UPS may vary significantly . Exosome-mediated secretion involves engulfing a portion of cytosol non-selectively into the so called intraluminal vesicles, whereas in autophagy-mediated secretion, cargoes are selectively imported into autophagosomes with the assistance of Hsp90 . By contrast, MAPS cargoes use the USP19-DNAJC5 chaperone cascade and perhaps other chaperones to gain access to late endosomes. Interestingly, recent studies reported yet another UPS pathway that exports mutated ER integral membrane proteins to the plasma membrane without passing through the Golgi . Intriguingly, the transport of misfolded pendrin to the plasma membrane in this type UPS is dependent on the HSC70 co-chaperone DNAJC14 , reminiscent of DNAJC5s involvement in MAPS. However, whether vesicles carrying these UPS cargoes first fuse with late endosomes/lysosomes prior to cargo delivery to the cell surface remains to be examined.
Multiple pathways to generate secretory late endosome or lysosomes
A Cytoskeleton And Axonal Transport
A constant interaction between microtubules and MAPs such as tau is a necessary element for axonal transport . Tau holds the microtubular tracks in place and plays a key role in their stability . When tau is subjected to hyper-phosphorylation, it loses the ability to bind to microtubules and to maintain their structure, causing tau aggregation into paired helical filaments and NFTs . The number of NFTs is linked to the degree of dementia, suggesting a correlation between NFT, dystrophic neurite formation and neuronal dysfunction . It seems that interrupting axonal transport will interrupt neuronal function and lead to eventual death .
Deposition of A plaques precedes tau phosphorylation and exerts a damaging effect upon the cytoskeleton giving rise to PHF formation. . Intraneuronal formation of A also happens prior to appearance of PHF, making it the upstream step in triggering the neurodegenerative events .
Don’t Miss: Parkinson Bicycle Cleveland Clinic
Antibody Detection Of Aggregation Markers In Parkinsons Disease
The accumulation of alpha-synuclein, one of the main structural components of Lewy bodies, is a hallmark of the latter stages of Parkinsons disease . Although its specific role in the neuronal degeneration process is not fully understood, studies have suggested that it is involved in aggregation of misfolded protein that eventually converges upon dopaminergic neurons in the form of Lewy bodies . Figure 2 demonstrates validation data for the Invitrogen alpha-synuclein antibody, which was tested using different parameters to ensure its specificity.
A growing number of genes associated with Parkinsons disease are related to mitochondrial function and oxidative stress, emphasizing the role of mitochondria in neuronal degeneration and disease progression . Similarly, impairment of lysosome-mediated protein degradation via autophagy or endocytosis directly impacts the ability of neurons to clear alpha-synuclein aggregates in Lewy bodies .
Figure 2. Assessment of alpha-synuclein antibody specificity. Immunofluorescence analysis in mouse brain tissue using alpha-synuclein polyclonal antibody and a neurofilament-L monoclonal antibody cells were counterstained with DAPI . Western blot analysis of alpha-synuclein antibody using CRISPR knockout of alpha-synuclein in HeLa cells and relative expression of alpha-synuclein in brain and liver. Western blot detection was performed as described in Figure 1.
| Target protein |
|---|
Tracking The Microtubule Cytoskeleton In Live Neurons
To visualize dynamic microtubules in live neuronal cultures, we offer the tubulin-selective fluorescent probes Invitrogen Tubulin Tracker Deep Red and Tubulin Tracker Green. These are cell-permeant fluorescent dyes that bind to polymerized tubulin within the microtubule. The Tubulin Tracker Green reagent has been used for the study of microtubule dynamics in Parkinsons diseaserelated research . Neuronal staining using the recently introduced Tubulin Tracker Deep Red reagent is shown in Figure 5.
Also Check: Similar To Parkinsons
The Influence Of Bacterial Amyloid On Alpha
Objective/Rationale: In Parkinsons disease , in nerve cells in the brain and in the intestine, alpha-synuclein proteins are misformed with abnormal folds and develop small toxic fibers. The reason why alpha-synuclein becomes misfolded in the brain or the gut is unknown. We hypothesize that alpha-synuclein misfolding in gut and brain neurons in caused by exposure to proteins of similar structure that are contained in bacteria that reside in the mouth and gut.
Project Description: Abnormal folding of alpha-synuclein has been described in aged pre-clinical models. We will establish three groups of middle-aged pre-clinical models, and expose them orally to bacteria that have amyloid proteins, which have a similar structure to misfolded alpha-synuclein. We will study the pre-clinical models for nine months as they age and develop stable colonies in the mouth and gut with amyloid-containing bacteria. Control bacteria that do not have the amyloid protein will also be used. We will evaluate the effects of oral administration of the amyloid-producing bacteria on growth of the bacteria in the gut, oral infections, markers in the blood for inflammation and free radicals, and development of misfolded alpha-synuclein in gut and brain neurons.
Lipid Homeostasis And Its Links With Protein Misfolding Diseases
- Centre for Misfolding Diseases, Department of Chemistry, University of Cambridge, Cambridge, United Kingdom
The maintenance of lipid homeostasis is essential for the normal functioning of living organisms. Alterations of the lipid homeostasis system remodel the composition of the lipidome, potentially leading to the formation of toxic lipid species. In turn, lipidome changes can affect the protein homeostasis system by causing perturbations that elicit protein condensation phenomena such as protein liquid-liquid phase separation and protein aggregation. Lipids can also be more directly involved the formation of aberrant condensed states of proteins by facilitating the early events that initiate these processes and by stabilizing the condensed states themselves. These observations suggest that lipid-induced toxicity can contribute to protein misfolding diseases, including Alzheimers and Parkinsons diseases. According to this view, an impairment of the lipid homeostasis system generates toxic states of lipids that disturb the protein homeostasis system and promote the formation of toxic states of proteins.
Recommended Reading: Prayer For Parkinson’s Disease
Models Combining A And Tau
Worm models
The link between A and tau pathologies in AD has remained incompletely understood, in part because animal models have not been particularly successful in recapitulating both features of the disease. Crossing C. elegans strains expressing A42 and an aggregation-prone tau mutant in neurons was shown to lead to an exacerbation of the toxic phenotype in terms of lifespan, viability of offspring, chemotaxis and other behavioural assays . Conversely, crossing an anti-aggregation tau variant and an A42 strain has not led to a significantly different phenotype compared to A42 alone. The total aggregation load as observed from Congo red staining was found to be much increased in the double-transgenic line with the pro-aggregation tau mutant, and signs of neurodegeneration and neuronal loss were observed, but a mechanistic link between A42 and tau toxicity has not been revealed from these studies.
Fly models
Rodent models
Taking advantage of the knock-in strategy described in the Section A models for the APP-based models, a recent study reports on the humanisation of the mouse MAPT gene . Although the subcellular distribution of human tau appears normal in this model, crossing it with the APP knock-in model, APPNLGF, results in enhanced tau phosphorylation at several sites. Tau tangles were not identified, yet these more physiological models may prove useful for further studies into the relationship between A and tau pathology.
Maps And Neurodegenerative Diseases
Unconventional protein secretion has long been thought to play a role in neurodegenerative diseases. Several neurodegenerative disease-associated soluble proteins are known to accumulate in extracellular space in pathologic tissues . The cause of their release from cells is largely unclear, neither is the underlying mechanism, but these proteins apparently need to be exported via an UPS mechanism since they do not possess any leader sequences. Intriguingly, some neurotoxic proteins can even undergo cell-to-cell transmission . These proteins are usually aggregation-prone. Thus, their transfer from cell to cell has been thought to facilitate the spreading of protein aggregates in diseases such as Alzheimers disease and Parkinsons disease . One sensible model in analogous to the prion hypothesis suggests that misfolded proteins upon entering recipient neurons may serve as seeds to cause more protein misfolding and aggregation . Accordingly, intercellular transmission of misfolded proteins is comprised of at least two steps: release of misfolded proteins from a donor neuron and their uptake by a recipient neuron. It is now well appreciated that neurons can use a variety of surface receptors to take up misfolded proteins from extracellular milieu . By contrast, little is known about how misfolded proteins lacking leader sequence are exported from cells.
Proposed models for intercellular transmission of misfolded proteins
Read Also: Does Sam Waterston Have Parkinsons
Er Stress And Upr In Pd
So far, the involvement of the UPR in PD has been demonstrated in the toxin-induced PD models of 6-hydroxydopamine , methyl-4-phenyl-1,2,3,6-tetrahydropyridine , and rotenone . Hoozemans and colleagues were the first to report the relationship between the activation of the UPR system and PD . They observed increased levels of phospho-R-like endoplasmic reticulum kinase and phospho-inositol-requiring kinase 1 -subunit proteins, as well as 78-kDA glucose-regulated protein , activating transcription factor 4 , and the transcription factor CHOP in SNpc from post mortem samples of patients with PD compared with controls . Nevertheless, activation of the UPR system in PD disease was confirmed with the accumulation of the protein-disulfide isomerase protein , a member of the disulfide isomerase family associated with disulfide bond formation, reduction, or isomerization of nascent proteins , which constitutes an adaptive and neuroprotective response against ER stress .
Effects Of Lipids On A Aggregation In Alzheimers Disease
Alzheimers disease is the most common cause of dementia, a condition that affects over 50 million people worldwide . At the molecular level, this disease is characterized by the presence of amyloid plaques, which are aberrant deposits formed primarily by the A peptide in the brains of affected individuals . According to the amyloid hypothesis, the formation of these deposits triggers a series of pathological events that ultimately result in synaptic loss and neuronal death . In a small fraction of cases, the aggregation of A is enhanced by familial mutations, causing the early onset of the disease . More commonly, however, the disease is associated with an age-related impairment of A homeostasis, which involves perturbations in A synthesis, trafficking and degradation . The deterioration of A homeostasis can also result from an altered cellular environment that becomes more conducive to A aggregation in ways that we are beginning to understand. In particular, lipidomic studies have revealed widespread disease-related changes in the human lipidome in Alzheimers disease, in particular in phospholipids, cholesterol, and triglycerides , and identified specific lipids as biomarkers of the disease, including in particular a set of phosphatidylcholine metabolites .
You May Like: Adaptive Silverware For Parkinson’s
Parkin Malfunction In Parkinsons Disease
Rebalancing The Proteostatic Network
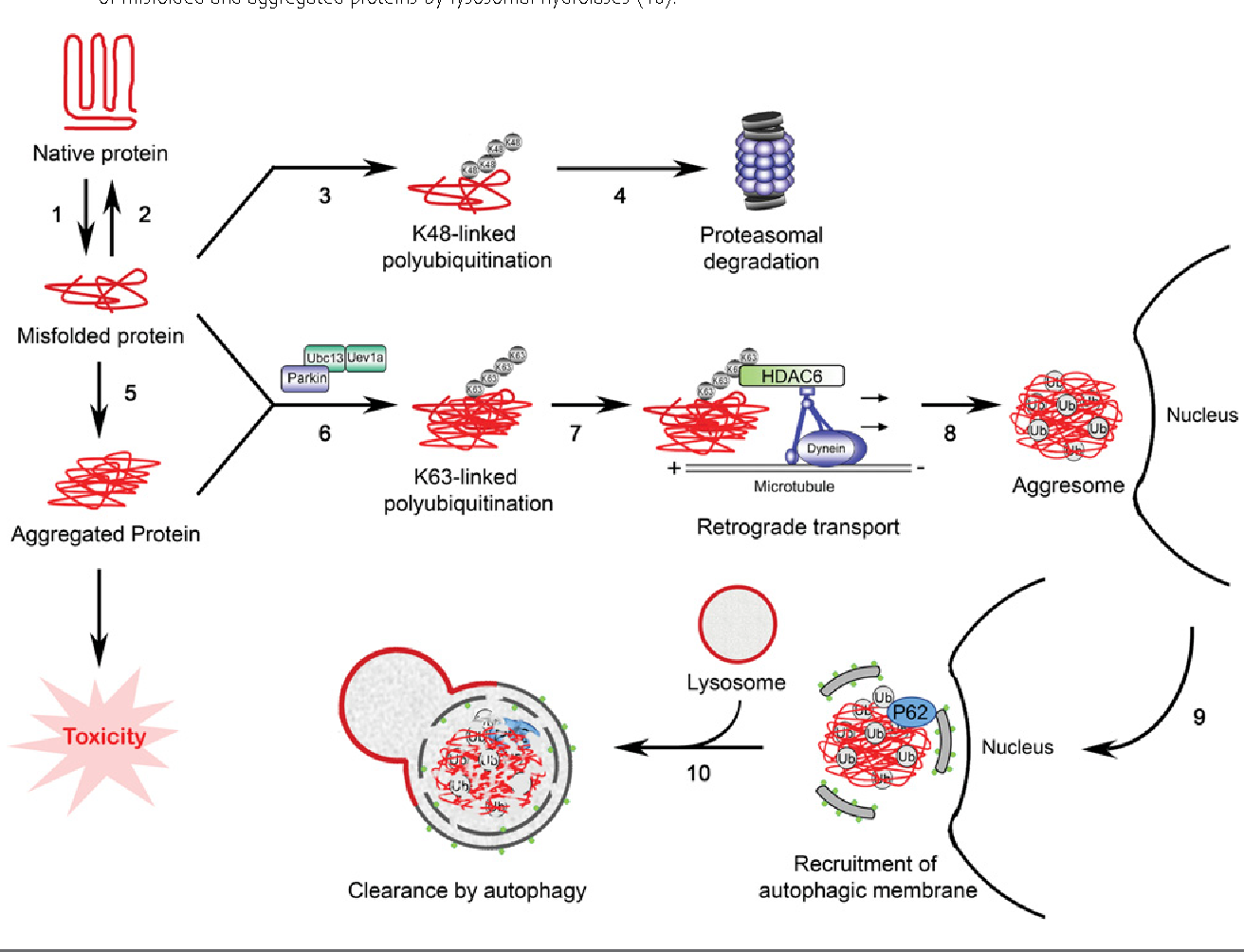
The ability of the cells proteostatic machinery to counter proteotoxic stressors deteriorates with age, and is further compromised by mutations and other disease conditions that lead to the accumulation of misfolded proteins . Thus, another potential approach to the development of therapeutics would involve large-scale rebalancing of the proteostatic network. Indeed, the efficacy of mTOR inhibitors may reflect their ability to provoke large-scale rebalancing of protein synthesis and degradation pathways. Another attractive target in this regard is heat shock factor 1 , a transcriptional activator that helps coordinate the heat shock response. The heat shock response diminish with age and in neurodegenerative disease . In addition, it was recently shown that HSF1 degradation is abnormally elevated in mouse and human -synucleinopathy . Over-expression of human HSF1 has been shown to be neuroprotective in cell models of neurodegenerative diseases , to reduce polyglutamine aggregate formation and prolong lifespan in a mouse model of HD and to reduce pathogenic androgen receptor accumulation and neurotoxicity in a mouse model of spinobulbar muscular atrophy . Small molecule activators of HSF1 have now been identified and shown to have neuroprotective effects in cell or animal models of neurodegenerative diseases .
Also Check: Sam Waterston Tremor
Effects Of Lipids On Protein Misfolding Liquid
Lipids can affect the conversion of proteins into the amyloid state by modulating the protein aggregation network in a variety of ways . In the deposition pathway, the protein aggregation process proceeds through a series of microscopic steps, which begin with the formation of small disordered aggregates through primary nucleation events in which monomeric proteins come together . These initial soluble aggregates convert into more ordered species, which can then grow into amyloid fibrils . In its homogeneous version, this process happens spontaneously in the absence of co-factors, while in its heterogeneous version, the various steps, including in particular the initial nucleation events, are triggered by cellular factors, which may include lipids, metal ions and metabolites .
More recently, it has been realized the phenomenon of liquid-liquid phase separation can open another route to the formation of amyloid assemblies . In this process, referred to as the condensation pathway in Figure 3, proteins initially phase separate reversibly into a dense liquid phase, which is associated with the formation of membraneless organelles. Under certain conditions, there could be primary nucleation events within the dense liquid phase, which can then give rise to the amyloid pathway. Also this process can be homogeneous or heterogeneous, depending on whether other cellular factors participate in it.
A And Apolipoprotein E
ApoE is a normal constituent of cells. In the nervous system, it acts as the main lipid transport protein with a wide variety of roles in intracellular signalling, immune modulation, glucose metabolism, lipid movement and lipoprotein metabolism . ApoE has been detected in the amyloid plaques in AD .
The ability of ApoE to interact with A, demonstrated its critical role in amyloid deposition and clearance . The apoE4 allele of ApoE is associated with high cholesterol in cardiovascular disease and particularly AD, however, the apoE2 allele confers some protection against hypercholesterolemia . ApoE2 and E3 formed stable complexes with A at levels of 20 fold greater than those occurring with apoE4 . The greater affinity of ApoE2 and E3 for A protects neurons from neurotoxic effects of A by facilitating the uptake of these complexes by apoE receptors. Conversely, apoE4 accelerates A deposition and progression/growth of A seeds to larger A plaques .
Also Check: Yopd Life Expectancy