Cognitive Deficits In Pd
PD patients experience a myriad of cognitive deficits, including, but not limited to, impairments in executive functions, language, memory, visuospatial skills, and dementia . To test for these deficits in mouse models of PD, primarily memory and general cognitive tests are used as it is hard to measure executive function in mice. The radial arm maze functions to assess the mouse’s ability to remember a set of spatial locations based on memory and not response patterns. The maze consists of eight arms and a central platform, with food rewards located randomly among the 8 arms . To increase motivation in learning the maze correctly, a 15% reduction in daily food intake is enforced. During the test sessions, four randomly selected arms are baited with one pellet of food each the baited arms are kept unchanged throughout the experiment . After each test session, the mice are evaluated for three parameters: working memory errors, reference memory errors, and total arm entries. Working memory errors are classified as reentries into baited arms that had been previously entered during the test session, and reference memory errors are classified as entry into non-baited arms . The mice were considered to have learned the task when the number of working memory errors reached zero and the number of reference memory errors is one .
Temporal And Spatial Glia Reactions After Mptp Intoxication
Systemic injections of MPTP result in a rapid onset of neuroinflammatory responses in the SN as well as in the CPu. These astroglia- and microglia-mediated responses are triggered by the impairment of mDA neuron function and maintenance, making the MPTP model suitable to analyse the associated neuroinflammatory changes . Due to its lipophilic nature and the ability to cross the BBB, the MPTP model has the advantage that microglia are not activated by direct injections or by mechanical manipulations of the central nervous system . This makes it superior to the 6-OHDA model, wherein the toxin has to be injected directly into the CNS parenchyma . Microglial reactions have been simply categorised into M1 and M2 reactions, which in theory, resemble two opposite activation states. While M1 activation is supposed to promote acute neuroinflammation, cellular and/or neurotoxicity, M2 activation is believed to mediate anti-inflammatory effects associated with tissue regeneration and wound healing. However, these theoretical M1/M2 activation states are the end-points of a microglia activation continuum. The influence of these activation states in neurodegenerative diseases are yet only partially understood, and need to be further addressed .
Worse Gi Symptoms Predict Anxiety Depression And Vice
Certain symptoms, like constipation, are known to precede by decades the onset of motor symptoms, and are linked with a poorer quality of life.
Studies suggest that gastrointestinal complications arise as a result of damage in the enteric nervous system , a network of nerve cells that independently regulates the gastrointestinal tract and sends signals to the brain. This damage is due to the accumulation of the alpha-synuclein protein.
Common treatments for constipation have proven ineffective in Parkinsons patients.
ATH434 is a small orally administered molecule designed to inhibit the aggregation of alpha-synuclein. In a mouse model of Parkinsons, treatment with ATH434 for four months prior to the onset of motor symptoms was reported to improve motor function and lessen abnormal alpha-synuclein clumps by redistributing labile iron in the brain.
Labile iron includes all forms of iron found within cells that are not associated with proteins. Known for its toxic potential, these forms of iron have been linked with mechanisms promoting the development of several neurodegenerative disorders, including Parkinsons.
Researchers investigated whether ATH434 could also lessen non-motor symptoms, such as GI dysfunction, in an animal model of Parkinsons.
They used the A53T mouse model, in which animals are modified to produce A53T alpha-synuclein, a mutated form of the alpha-synuclein protein associated with the disease in people.
Don’t Miss: How Long Does A Person Live With Parkinson’s Disease
Transgenic Animal Models Of Pd
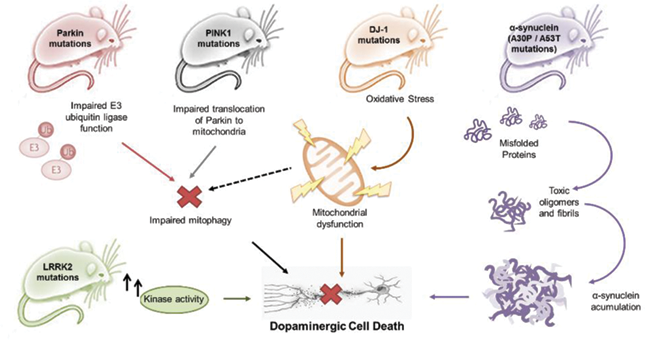
Although toxic models of PD have provided an invaluable bulk of information on disease pathology, the lack of an age-dependent, slowly progressive lesion and the fact that LBs are typically not observed in these models represent major drawbacks. The discovery of monogenic Mendelian forms of PD has provided considerable insights into the disease pathogenesis and the recent burst of genomewide association studies has provided evidence that familial and sporadic forms of PD may share common genetic backgrounds. These considerations have prompted the development of new animal models, in particular mice, which reproduce known PD-related mutations. Below, we describe transgenic mice models, as well as alternative models developed in non-mammalian organisms, such as Drosophila, Caernorhabditis elegans and zebrafish , which recapitulate monogenic mutations observed in familial PD patients. A list of known genes and related protein products involved in familial PD is provided by Alberio et al. ].
Growth Factors And Neurotrophic Factors
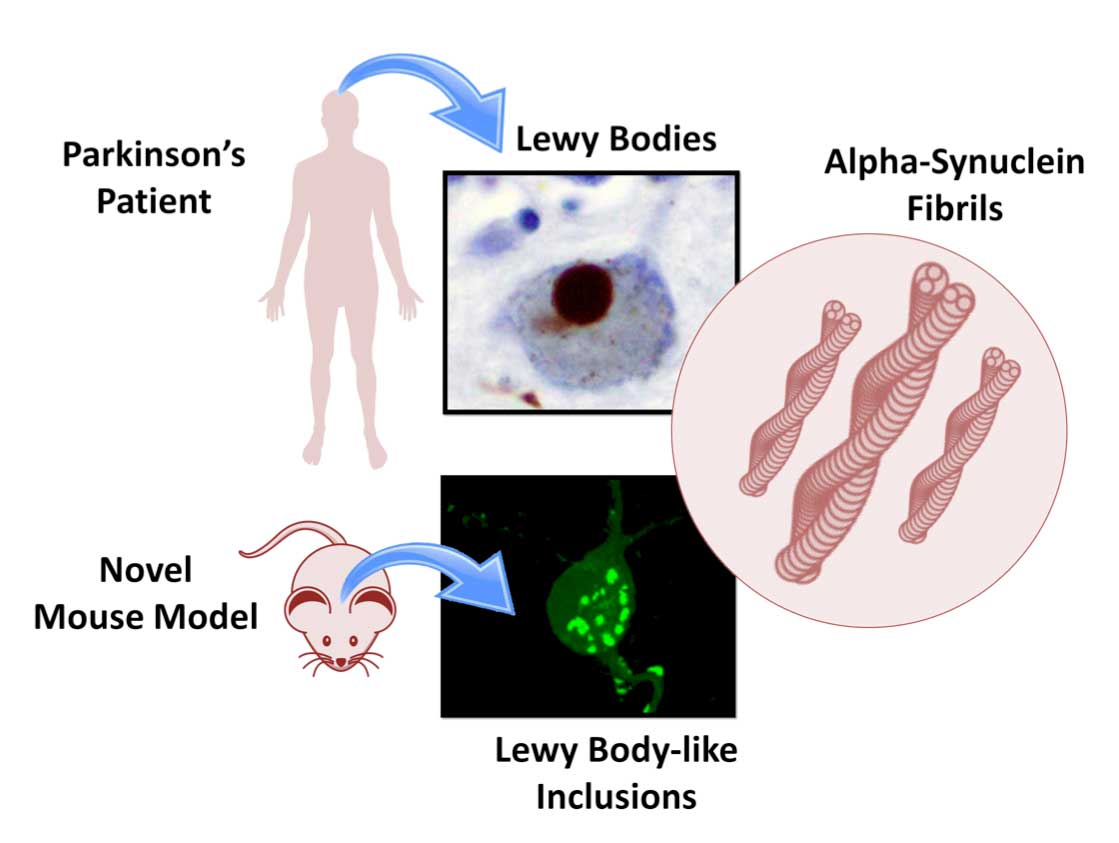
Neurotrophic factors are secreted proteins that play vital roles during development of the nervous system by influencing the maintenance of selective neuronal populations and supporting their survival and maturation through adulthood. Based on these properties, the potential of several neurotrophic factors has been investigated in the parkinsonian brain with the help of animal models and clinical trials . Here, we provide an update of the neurotrophic factors used in the MPTP mouse model, with a focus on neuroprotection, and the role of glia in these neurodegenerative processes.
Igfs are essential trophic factors required for the growth and development of the embryonic brain, with neuromodulatory functions in the adult brain . The protein encoded by the gene Igf-1 is served by its receptor Igf-1r, a transmembrane tyrosine-kinase receptor homologous to the insulin receptor. Mice haploinsufficient for the Igf-1r administered with MPTP show exacerbated losses of mDA neurons as compared to their WT littermates. Moreover, the Igf-1r+/ mice exhibit an increased microglial inflammatory response to the neurotoxin and a down-regulation of several anti-inflammatory pathways under control conditions, indicating that the Igf signalling pathway/s can reduce neuroinflammatory responses and sensitivity of mDA neurons to MPTP-induced inflammation .
Don’t Miss: What Is The Average Age For Parkinson’s Onset
Western Blot And Fractionation Of
One brain hemisphere per mouse was cut to small pieces on ice. Fractionation and lysis were done as previously described, with four washing steps of the 1% Triton X-100 insoluble pellet in 1 ml lysis buffer. Protein concentrations were measured with BCA kit using standard procedures . Western blots were run using standard methods with a maximum of 14 g protein per well. Antibodies used for the detection of -syn were mouse anti–syn- and rabbit anti-pS129–syn . Species-specific proteins were distinguished by molecular weight. Images were acquired in Imagelab version 5.2.1 and quantified in Fiji-ImageJ. Methods are described in detail in the .
Parkinsons Disease Models: An Overview Of Cellular And Animal Models For Neurodegeneration Research
Christina Korgiopoulou | January 29th, 2020 |
For centuries, animals have been used in biological research and helped scientists understand the mysteries of life. Experiments on animals have enhanced understanding of anatomy, physiology and pathology and led to great discoveries in medicine1. Parkinsons Disease is a common neurodegenerative disorder that is becoming more prevalent with aging populations. Because PD is a complex, heterogeneous disease with diverse clinical and pathological features, PD research involves a variety of disease models.
Don’t Miss: Pump For Parkinson’s Disease
Progressive Accumulation And Aggregation Of
First, we investigated the accumulation and aggregation of human -syn in this model. We showed a significant increase in the fluorescence density of -syn-GFP and of p–syn as an indicator of aggregated -syn in the striatum of TG mice between 3 and 13 months indicating a progressive accumulation and aggregation of human -syn . There was a trend that did not reach significance in the fluorescence density of these markers between age 3 and 8 months.
Figure 1
Discovery In Users Of Illicit Drugs
The neurotoxicity of MPTP was hinted at in 1976 after Barry Kidston, a 23-year-old chemistry graduate student in , US, synthesized MPPP with MPTP as a major impurity, and self-injected the result. Within three days he began exhibiting symptoms of Parkinson’s disease. The National Institute of Mental Health found traces of MPTP and other pethidineanalogs in his lab. They tested the substances on rats, but due to rodents’ tolerance for this type of neurotoxin nothing was observed. Kidston’s Parkinsonism was treated with levodopa but he died 18 months later from a cocaine overdose. Upon autopsy, Lewy bodies and destruction of dopaminergic neurons in the substantia nigra were discovered.
In 1983, four people in Santa Clara County, California, US, were diagnosed with Parkinsonism after having used MPPP contaminated with MPTP. The neurologist J. William Langston in collaboration with NIH tracked down MPTP as the cause, and its effects on primates were researched. After performing neural grafts of fetal tissue on three of the patients at Lund University Hospital in Sweden, the motor symptoms of two of the three patients were successfully treated, and the third showed partial recovery.
Langston documented the case in his 1995 book The Case of the Frozen Addicts, which was later featured in two productions by PBS, re-aired in the UK on the BBC science series Horizon.
Recommended Reading: Vascular Parkinsonism And Cognitive Impairment
The Human Lrrk2 G2019s Targeted Replacement Mouse
- The G2019S alpha-mutation is linked to the development of Parkinson’s disease, both sporadic and familial.
- In patients, the G2019S LRRK2 mutation is an autosomal dominant mutation that leads to pathology similar to what is observed with idiopathic Parkinson’s disease.
- At 18 months of age, LRRK2 G2019S mice show an increased locomotor response after amphetamine challenge.
- This knock-in mutation does not appear to affect basal motor function or more complex behavior in C57Bl6 mice.
The Rab29 Overexpression Mouse
- Rab29 is a Rab GTPase that acts as a key regulator in vesicle trafficking and maintaining the endosome-trans-Golgi network structure. Rab29, together with LRRK2, plays a role in the retrograde trafficking pathway for recycling proteins.
- Rab29 is one of five genes located within the PARK 16 locus that has been linked to Parkinson’s disease.
- Rab 29 is a direct substrate of LRRK2 a kinase protein in which autosomal dominant missense mutations are linked to Parkinson’s disease through increased kinase activity. In addition, Rab29 is a regulator of LRRK2, controlling its localization and activation.
- There is a substantial increase in Rab29 protein levels by western blot in brain, kidney, and distal colon, with a moderate increase in spleen, lungs, macrophages.
Read Also: Green Tea For Parkinson’s
Current Mouse Models For Pd
PD mouse models are expected to closely match human pathology. The most important signs to replicate are motor symptoms, Lewy body formation, neuronal cell loss in the basal ganglia, age-related disease progression, and nonmotor symptoms. None of the current models satisfies all of these criteria. However, many models fulfill a subset. Empirical choices of adequate models to answer specific questions in translational research need to consider, case by case, the model-specific advantages and limitations.
Parkinson’s Disease Mouse Model Resource
Improved animal models of Parkinsonism are essential to advance our understanding of disease pathophysiology and for eventual testing of potential therapeutics. To that end, the Michael J. Fox Foundation for Parkinsons Research has funded the generation, characterization and distribution of animal models of Parkinsonism as part of its strategy to provide preclinical tools to the research and drug development community.
We are currently characterizing novel models side-by-side with some of the more prominent existing mouse models of PD. Unlike most PD models developed to date, these will be available to pharmaceutical and biotechnology companies for use to develop and test potential therapies. Also available are “research tool” strains including transgenics that express marker genes in affected brain regions, and strains with systems for regulating gene expression in specific types of neurons.
JAX® Mice useful to study Parkinsons disease
The WORM Human OrthoLogy Explorer is a meta-tool that uses machine learning to predict novel least diverged orthologs by integrating ortholog predictions from 17…
- Research HighlightNovember 10, 2021
- Profile StoryNovember 09, 2021
You May Like: Is Thumb Twitching A Sign Of Parkinson’s
Rebuttal From Roger A Barker
In his defence of the usefulness of animal models for studying PD, Anders Björklund lays out the history of how such models came into existence and their utility over the years. This includes an illuminating discussion on how L-dopa came into clinical use in PD despite very limited preclinical work in flawed animal models. Indeed, the critical work showing the value of this whole approach came from early experimental medicine trials in patients. These trials initially showed no benefit for this agent in PD , but through an iterative process this therapeutic turned into, and has remained, the mainstay of managing PD. Thus, we can see that the single biggest breakthrough in the treatment of PD was essentially done independently of any animal models.
So, I come back to where I began in my initial arguments against using animal models for PD to predict clinical effects and therapies. Namely I would advocate that more targeted iterative experimental medicine approaches are now needed to better treat PD just as was done in the 1960s!
Animal Models Have Not Failed Us: Anders Bjrklund
Can we do without animal models in PD research? Investigators involved in the development of new therapies and treatments are rightly concerned about the relevance and predictability of disease models for the initiation and design of clinical trials. This distrust is understandable given the numerous examples where seemingly convincing animal data have not panned out in subsequent clinical trials. The experience from the stroke field is particularly disheartening. This is even more disturbing since, in this clinical condition the animal models seem as perfect as they can be: the ischemic insults used in the animal experiments are identical to the ones seen in patients and should thus have a high level of predictability. Nevertheless, there are many cases where an intervention with a striking and convincing treatment effect in stroke models has failed when applied to patients. These failures have not only been extremely costly for the industry but they have also been discouraging and fostered a cynical attitude toward the need of animal models for the development of new therapies: If the models are misleading and lack predictability we will do better without them.
You May Like: Reishi Mushroom Parkinson’s Disease
Cellular Models Of Parkinsons Disease
Cell cultures like primary dopaminergic neurons, mesencephalic slice cultures and immortalized cell lines are used as models in PD research. Compared to animal models, cellular models are advantageous because they generate pathology quickly, are inexpensive, and do not have the same ethical concerns involved with animal models. They can reproduce dopaminergic neuron degeneration and alpha synuclein aggregation but usually results from cultured cells ultimately need to be validated in animals. The in vitro models provide useful information on the PD pathogenesis and are useful tools in drug development4,5.
Animal Models Of Parkinsons Disease: Are They Useful Or Not
Article type: Position Paper
Authors: Barker, Roger A.a * | Björklund, Andersb *
Affiliations: Department of Clinical Neuroscience and WT-MRC Cambridge Stem Cell Institute, Forvie Site, Cambridge, UK | Department of Experimental Medical Science, Wallenberg Neuroscience Center, Lund University, Lund, Sweden
Correspondence: Correspondence to: Roger A. Barker, Department of Clinical Neuroscience and WT-MRC Cambridge Stem Cell Institute, Forvie Site, Cambridge CB2 0PY, UK. Tel.: +44 1223 331160 Fax: +44 1223 331174 E-mail: Anders Björklund, Department of Experimental Medical Science, Wallenberg Neuroscience Center, Lund University, 22184 Lund, Sweden. Tel.: +46 462220540 E-mail: .
Keywords: Animal models, drug discovery, experimental therapies, alpha-synuclein, dopamine, Lewy bodies
DOI: 10.3233/JPD-202200
Journal: Journal of Parkinson’s Disease, vol. 10, no. 4, pp. 1335-1342, 2020
Abstract
The use of animal models in Parkinsons disease research has been controversial in terms of how well they relate to the clinical condition and thus their utility for translating therapies from the lab to the clinic. In this article, two researchers debate this issue with Roger Barker taking the view that such models are not useful and may even be misleading, while Anders Björklund defends their use and highlights their value in better understanding and treating this condition.
Recommended Reading: Can You Have Parkinson’s Without Shaking
Neurotoxin Models Of Parkinsons Disease
Neurotoxin- based models produced by 6-hydroxydopamine , 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine , rotenone and paraquat are widely used because of their ability to cause oxidative stress that leads to dopaminergic neuronal death10. These models have been very useful and valuable tools as they are easily made and mimic the symptoms of PD like motor deficits, but do not produce its pathological features. The main drawback of this model is that induces rapid neurodegeneration causing motor deficits which is contradictory to the natural progression of the disease in humans11. PD is a chronic neurodegenerative disorder and the degeneration of the dopaminergic neurons in the substantia nigra can start years before the first motor symptoms develop.
Preclinical Models Of Pain In Parkinsons Disease
From the outset, it is important to note that we are not considering here animal models of pain per se. The existence of good models in the pain field is itself subject to ongoing debate and outside of the scope of this review., Here, we are specifically interested in whether, and how, animals can be used to model pain in PD. As such, our attention is focused solely on existing animal models of PD and how effectively they recapitulate what is seen with respect to pain in the clinical setting.
Studies investigating pain in animal models of PD have so far almost all been conducted in rodents: no data are available regarding pain phenotypes in non-human primate models of PD or those constructed in multicellular model organisms like zebrafish, C. elegans or drosophila. In the remainder of this review, we discuss the various rodent models of pain in PD, considering how well each exhibit face and predictive validity and how they have helped progress the understanding of pain in PD. Ultimately, it is hoped that one or more of these models might provide an accepted testbed for use in the search for novel analgesics to better treat pain in PD.
You May Like: Can Parkinson’s Tremors Come And Go