Active Immunotherapy Shows Safety Tolerability In Parkinsons
Sustained immunogenicity seen in phase I study
The first study of active immunotherapy against -synuclein in patients with Parkinsons disease showed safety and sustained immunogenicity.
PD01A, a short peptide immunotherapy that targets oligomeric -synuclein in PD, met safety and tolerability goals in a phase I study, reported Gunther Staffler, PhD, of the pharmaceutical company AFFiRiS in Vienna, Austria, and coauthors in Lancet Neurology.
Active immunotherapy elicits a self-produced immune response which is sustained over time. Passive immunotherapy involves regular infusions of in vitro-produced monoclonal antibodies.
In 21 patients treated with PD01A who formed four groups depending on two sequential randomizations to 15 g or 75 g, all had at least one adverse event. Most were considered unrelated to study treatment except for transient local injection site reactions, which affected all but one participant.
Systemic adverse events that were potentially treatment related included fatigue , headache , myalgia , muscle rigidity , and tremor .
The first randomization led to four subcutaneous priming immunizations with either 15 g or 75 g and 52 weeks of follow-up. The geometric group mean titer of antibodies against the peptide increased from 1:46 at baseline to 1:3,580 at week 12 in the 15 g dose group. In the 75 g dose group, corresponding titers went from 1:76 to 1:2,462.
Limitations of the study include absence of a placebo group and small sample size.
Immunotherapy Promising In Parkinson’s Disease
Pauline Anderson
VANCOUVER Immunotherapy using a novel monoclonal antibody appears to be a promising new approach to treating Parkinson’s disease .
A phase 1b study of a humanized IgG1 monoclonal antibody directed against -synuclein showed that it was well tolerated, had an acceptable safety profile, and penetrated the central nervous system .
Importantly, the agent reduced levels of serum -synuclein by up to 97% after a single dose, the researchers found.
“The antibody targets the toxic, aggregated, synuclein and neutralizes it,” lead author, Joseph Jankovic, MD, professor, neurology, and distinguished chair in movement disorders, Baylor College of Medicine, Texas, told Medscape Medical News.
Dr Jankovic presented the new findings here at the International Congress of Parkinson’s Disease and Movement Disorders 2017.
“In my opinion, it was the hottest topic during therapeutic sessions at that meeting,” he said.
-Synuclein is a protein found in Lewy bodies of patients with PD. It is thought to be involved in degenerative neuronal death.
In neutralizing extracellular neurotoxic forms of -synuclein, PRX002 may inhibit cell-to-cell transmission of the aggregated, pathogenic form of -synuclein, thereby modifying disease progression associated with PD.
The double-blind study included 80 mostly white male patients with mild to moderate PD who were receiving stable anti-PD treatments.
The treatment was administered in three intravenous infusions about 28 days apart.
Symptoms Resembling Parkinsons Disease Are A Cautionary Finding In Car
Mount Sinai scientists have become the first to report a potentially serious side effect related to a new form of immunotherapy known as CAR-T cell therapy, which was recently approved for the treatment of multiple myeloma. Their findings were published as a case study in Nature Medicine in December.
Multiple myeloma is a complex and incurable type of blood plasma cancer that often requires multiple treatments as the disease progresses and becomes resistant to previous therapies, often resulting in chronic disease with periods of acute illness.
CAR-T cell therapy uses genetically engineered immune system cells known as chimeric antigen receptor T cells. In the specific version at issue, the CAR-T cells were used to target a protein known as B cell maturation antigen . BCMA is commonly found in multiple myeloma, and this therapy has shown impressive response rates in people with particularly complex, treatment-resistant multiple myeloma.
Our findings will impact the risk-benefit assessment of BCMA-targeted CAR-T cell therapy for multiple myeloma and have already led to improved monitoring and proactive management of neurologic adverse events across clinical trials of BCMA-targeted therapy, said Oliver Van Oekelen, MD, PhD student at the Icahn School of Medicine at Mount Sinai and the first author of the manuscript.
Don’t Miss: Does Parkinson’s Cause High Blood Pressure
Future Perspectives And Conclusions
Through detailed studies on patients, but also in animal models of PD, key neurodegenerative pathomechanisms of this disease have been decrypted. In addition to the damage to neuronal cells, it is now obvious that also glial cells are affected and that a strong neuroimmunologic interaction takes place with aSyn being the central player. From todays perspective, a modulation of this cross-cell disease propagation in PD appears possible by targeting of aSyn.
Very elegant neuroimmunologic tools have been developed to inhibit disease spread by extracellular aSyn. The elimination of aSyn by targeted antibody-based technologies with active or passive immunization therefore seems very pragmatic. Certainly, technologic challenges, such as overcoming the blood-brain barrier or the targeted elimination of only aggregated aSyn, still need to be optimized. However, we are well on the way to providing answers to these questions as several human clinical trials with ambitious expectations are currently underway. The simultaneous application of antibody-based therapies in atypical Parkinsons syndromes such as PSP or also Alzheimers disease will in any case result in important basic insights. This will, it is our firm conviction, enable new technologic advances in the immunotherapy for PD and bring about new powerful options to modify disease progression.
Cancer And Parkinson’s Disease: Common Targets Emerging Hopes
School of Health and Life Sciences, Teesside University, Middlesbrough, United Kingdom
National Horizons Centre, Teesside University, Darlington, United Kingdom
*Correspondence to: Prof. Dr. Tiago F. Outeiro, Department of Experimental Neurodegeneration, University Medical Center Göttingen, Waldweg 33, 37073 Göttingen, Germany E-mail: or Dr. Panagiota S. Filippou, School of Health and Life Sciences, Teesside University, Middlesbrough TS1 3BX, United Kingdom E-mail:
Department of Experimental Neurodegeneration, Center for Biostructural Imaging of Neurodegeneration, University Medical Center, Göttingen, Germany
Cluster of Excellence Multiscale Bioimaging: From Molecular Machines to Networks of Excitable Cells , University of Göttingen, Göttingen, Germany
Max Planck Institute for Experimental Medicine, Göttingen, Germany
Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, Newcastle upon Tyne, United Kingdom
*Correspondence to: Prof. Dr. Tiago F. Outeiro, Department of Experimental Neurodegeneration, University Medical Center Göttingen, Waldweg 33, 37073 Göttingen, Germany E-mail: or Dr. Panagiota S. Filippou, School of Health and Life Sciences, Teesside University, Middlesbrough TS1 3BX, United Kingdom E-mail:
School of Health and Life Sciences, Teesside University, Middlesbrough, United Kingdom
National Horizons Centre, Teesside University, Darlington, United Kingdom
Read Also: Michael J Fox Parkinson’s Website
Immunotherapies For Neurodegenerative Diseases
- 1Neuroscience Research Center, Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon
- 2Department of Biomedical Sciences, University of the Pacific, Arthur Dugoni School of Dentistry, San Francisco, CA, United States
- 3College of Health Sciences, Abu Dhabi University, Abu Dhabi, United Arab Emirates
The current treatments for neurodegenerative diseases are mostly symptomatic without affecting the underlying cause of disease. Emerging evidence supports a potential role for immunotherapy in the management of disease progression. Numerous reports raise the exciting prospect that either the immune system or its derivative components could be harnessed to fight the misfolded and aggregated proteins that accumulate in several neurodegenerative diseases. Passive and active vaccinations using monoclonal antibodies and specific antigens that induce adaptive immune responses are currently under evaluation for their potential use in the development of immunotherapies. In this review, we aim to shed light on prominent immunotherapeutic strategies being developed to fight neuroinflammation-induced neurodegeneration, with a focus on innovative immunotherapies such as vaccination therapy.
Neuroinflammation And Microglia Activation In Parkinsons Disease
A recent ground breaking paper, that discovered lymphatic vessels in the brain, highlights the role for the immune system in the brain . Microglia and astrocytes are the resident immune cells of the brain, and in this capacity they are likely to play crucial roles in the success of immunotherapies forneurodegenerative diseases. In PD, these cells are activated, as part of a neuroinflammatory response, implicating that the immune system plays a role in the pathogenesis of PD. However, observations of activated microglia and astroglia do not clarify whether neuroinflammation is the primary or secondary event in PD pathogenesis . Studies in PD patient brains have revealed neuroinflammation particularly in the basal ganglia and neocortex . The microglia in these regions exhibit an activated morphology, and in the substantia nigra they surround the degenerating dopaminergic neurons . They may consist of a mixed population of invading peripheral monocytes who act in concert with resident microglia . The adaptive immune response also plays a role with the substantia nigra of PD patients exhibiting a 10-fold increase in numbers of CD4+ and CD8+ T-lymphocytes compared to controls . Further support for inflammation being important in PD pathogenesis comes from studies showing that PD is associated with certain HLA-variants and that intake of some non-steroidal anti-inflammatory drugs reduces the risk of developing PD .
Don’t Miss: Unified Parkinsons Disease Rating Scale
Glucagonlike Peptide 1 Receptor Agonists And Other Antidiabetic Agents
Biological processes involved in PD share common features with obesity and type 2 diabetes mellitus , including the dysregulation of insulin signaling in the brain. The term brain insulin resistance has been suggested to describe decreased sensitivity of CNS pathways to insulin, followed by disturbances in synaptic, metabolic and immune response functions . Strategies to normalize insulin sensitivity in neurons have thus been in the spotlight of clinical trials aiming to establish whether they may provide neuroprotective actions.
The neuroprotective effect of GLP-1 RAs is assumed to be mediated by improved brain insulin sensitivity however, human studies evaluating their biological effect in the CNS are limited. Functional MRI imaging studies have primarily focused on investigating brain networks involved in the anorectic effect of GLP-1 RAs , but sparse mechanistic data are available for understanding neuroprotective effects of these drugs. In a more recent trial of exenatide in PD, disease modifying effects measured by nigrostriatal dopamine transporter imaging were reported . Subsequently, brain insulin and Akt signaling pathways were also evaluated in neuronal-derived exosomes and it was shown that exenatide treatment, but not placebo, activated these pathways . This significant, secondary analysis of the trial increases understanding of the molecular mechanism underlying the treatment effect and provides a possible biomarker to measure target engagement.
New Immunotherapy Could Stop Progression Of Parkinsons Disease
A new form of immunotherapy offers promise as potentially the first treatment to slow or stop the progression of Parkinsons disease. Although its effectiveness still must be determined in further clinical trials, new research published today shows that it meets the fundamental principle of medicine: First, to do no harm.
The study, published today in JAMA Neurology, found that clinical trial participants safely tolerated even high doses of the treatment, an investigational drug called PRX002/RG7935.
OHSU is one of several sites recruiting participants into phase II of the clinical trial for that drug, which will further study safety and tolerability as well as measure the potential effectiveness of the therapy over a year. The randomized controlled trial will use a standardized scale for Parkinsons to measure the progression of the disease among participants given a lower dose, higher dose and placebo. Researchers are looking for participants who have been recently diagnosed with Parkinsons.
This drug has the potential to have the greatest effect for people early in the disease, within two years of diagnosis, co-author Joseph Quinn, M.D., professor of neurology in the OHSU School of Medicine and director of OHSUs Parkinson Disease and Movement Disorders Program.
This study was sponsored by Prothena Biosciences Limited of Dun Laoghaire, Ireland.
You May Like: Wrist Weights For Parkinson’s Tremors
Biomarkers And Immunotherapy For Parkinson’s Disease
Objective/Rationale:We theorize that the interplay between the immune system and the brain plays a substantive role in the progression of Parkinsons disease. Moreover, we posit that the immune system becomes dysfunctional as a result of the continuous presence of misfolded, aggregated, and nitrated proteins released from dead or dying nigral neurons and present in the lymphoid system. The deficit occurs in a class of lymphocytes present in blood and as such may be used as a biomarker for disease. Correcting the deficit may also be a target for therapy.
Project Description:The project will involve collecting blood specimens from Parkinsons disease patients and analyzing specific subsets of lymphocytes by state of the art immunological assays. Such an analysis will allow the determination of specific immune deficits that would occur in disease. Comparisons with patient care givers matched by age and demographics would serve as controls. A comprehensive set of functional assays for blood cells will be performed to precisely define any/all deficit and the means to correct it. Such an analysis could yield new sets of biomarkers to stage disease and its progression with an eye towards future investigations towards determining whether correction of the dysfunction would improve disease outcomes.
Current Experimental Immunotherapies Targeting T
Since the T-cell response to Syn-derived antigens has been extensively involved in the development of neuroinflammation and consequent neurodegeneration in Parkinsons disease, many researchers have attempted to attenuate this T-cell response as a therapeutic approach to stop the disease progression. Some studies have sought to decrease the inflammatory response induced by Th1 and Th17 cells, others have attempted to potentiate the immunosuppressive response of Treg, and other researchers have tried to do both.
To inhibit the inflammatory T-cell response involved in Parkinsons disease, a group of studies has tested the therapeutic potential of the well-known immunosuppressive drug FK506 , which has been broadly used to inhibit the graft rejection. Its immunosuppressive effect lies in the binging to the immunophilin FK506-binding protein to form the FK506-FKBP complex, which inhibits calcineurin activity, thus attenuating T-cell activation . This immunosuppressive drug has shown therapeutic effects reducing the number of T-cells infiltrated into the SN, and attenuating the motor impairment and the neurodegeneration in a rat model of Parkinsons disease induced by the unilateral stereotaxic delivery of AAV encoding for the A53T hSyn . FK506 has also shown to protect from the reduction of dopamine contained in the striatum in the mouse model of neurodegeneration induced by MPTP .
Experimental T-cell-based immunotherapies tested in pre-clinical models of Parkinsons disease
Don’t Miss: Can Parkinson’s Go Away
Can Immunotherapy Offer Hope For Parkinsons Sufferers
Despite decades of research, effective treatments for Parkinsons disease are proving elusive. Could immunotherapy finally provide a solution?
More than seven million people worldwide are currently living with Parkinsons disease, an incurable neurodegenerative disorder. Current treatment options are limited as they only attempt to mask the symptoms rather than address the cause.
But there is hope on the horizon. Multiple biotech companies are developing a range of immunotherapies to slow down or even completely halt the progression of Parkinsons.
Researchers believe the main culprit is a toxic form of a protein called alpha-synuclein, which accumulates in the brain over time due to mutations in the gene that encodes the protein. As clusters of alpha-synuclein form inside neurons, they cause the characteristic Parkinsons symptoms, ranging from tremors to muscle stiffness. Eventually, the neurons die.
Scientists have been designing antibodies that specifically target and bind to the toxic form of alpha-synuclein and remove it from the body.
The belief is that if you can reduce the levels of this toxic species, you can slow down the progression of the disease, Gunilla Osswald, CEO of Stockholm-based BioArctic, told me. This is what weve seen in preclinical models using mice. Our antibody makes the motor symptoms come much later, and they survive far longer.
Summary: The Main Takeaways
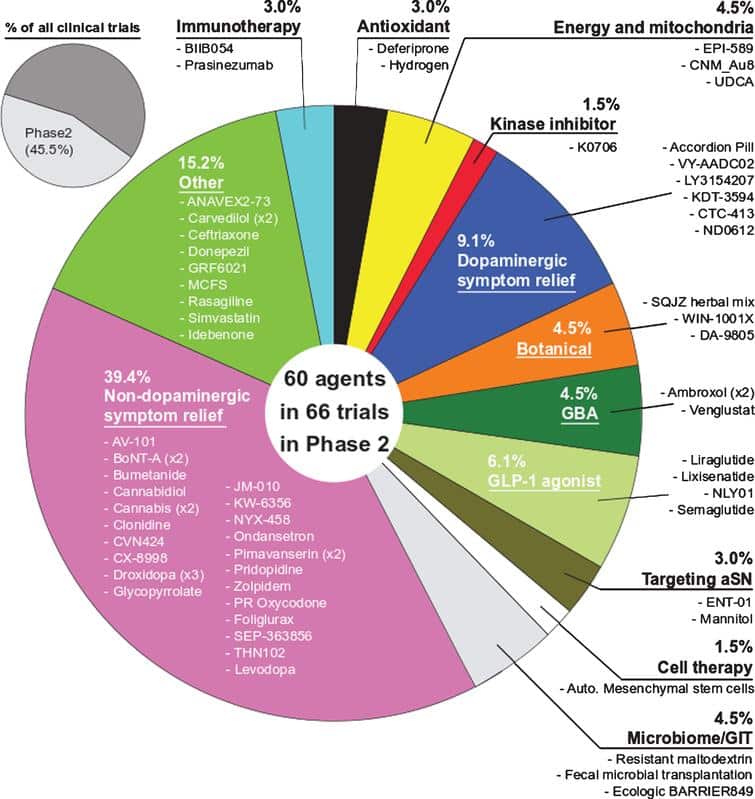
The amount of research being conducted is a very positive sign, and it suggests that 2022 will be an extremely productive year for Parkinsons research. The breadth of approaches now being applied to Parkinsons is also very encouraging, and in the next few years we may have answers to some fundamental questions regarding the biology that may be underlying many cases of PD .
There is certainly going to be a lot of clinical trial results being announced in 2022! The top 5 clinical trial results that I will be looking out for this year are:
- The Phase II UP study results evaluating UDCA in Parkinsons
- The Phase II Lixisenatide study results more data on GLP-1R agonists in PD
- The Phase II Liraglutide study results even more data on GLP-1R agonists in PD
- The Phase II Peptron study results lots of data on GLP-1R agonists in 2022
- The Phase II Deferiprone study results addressing an important question
And with bulging pockets, we can expect more acquisitions and partnering deals to come in 2022. Any fears that big pharma companies are quitting neurodegeneration appear to be misplaced with aging demographics in the Western world, the market opportunities are simply too big for these enormous companies to ignore.
All of this collective activity provides encouraging signs for future research focused on finding new therapies for slow, stopping and reversing Parkinsons.
But now its time to give the fingers a wee rest.
About the author
Footer example header
Read Also: Amino Acids And Parkinson’s
Component #3 Some Form Of Restorative Therapy
Once the condition has been slowed/halted and a neuroprotective/nurturing environment is in place to protect the remaining cells , a curative treatment for Parkinsons will require replacing some of the cells that have been lost.
And until we have developed methods that can identify Parkinsons long before the motor features appear , some form of cell replacement therapy is required to introduce new cells to take up lost function.Cell transplantation currently represents the most straight forward method of cell replacement therapy.
Cell Transplantation
Traditionally, the cell transplantation procedure for Parkinsons has involved multiple injections of developing dopamine neurons being made into an area of the brain called the putamen . These multiple sites allow for the transplanted cells to produce dopamine in the entire extent of the putamen. And ideally, the cells should remain localised to the putamen, so that they are not producing dopamine in areas of the brain where it is not desired .
Targeting transplants into the putamen. Source: Intechopen
Transplanted dopamine neurons. Source: Sciencedirect
The transplanted cells take several years to develop into mature neurons after the transplantation surgery. This means that the actually benefits of the transplantation technique will not be apparent for some time . Once mature, however, it has also been demonstrated that these transplanted cells can produce dopamine.
Source: Fujifilm
Ok, thats it.
I think we are done.