Perturbations Of Dopamine Homeostasis By Excessive Gdnf Overexpression
DA is submitted to an intricate network of regulations controlling its homeostasis . Firstly, DA regulates its own synthesis by binding to TH and retro-inhibiting its activity . After release in the synaptic cleft, DA is rapidly re-uptaken by the dopamine transporter . Finally, binding to the pre-synaptic D2R autoreceptor results in a negative feed-back on DA synthesis and release whereas binding to D1R-type receptor on striatal medium spiny projection neurons provides a long-loop retro-control .
GDNF interferes with DA homeostasis at different levels . It increases DA available in the synaptic cleft via different mechanisms such as: stimulation of TH phosphorylation, which blocks the DA binding site and thus reduces the retro-inhibition of TH activity GDNF enhancement of DA release via inhibition of a A type K+ channel thus provoking depolarization and Ca2+ entry reduction of DAT activity via Ret/DAT interaction . These effects can ultimately lead to compensatory mechanisms such as downregulation of TH transcription .
Table 1. GDNF-induced neurochemical changes in non-lesioned dopaminergic system.
Cerebral Dopamine Neurotrophic Factor And Mesencephalic Astrocyte
CDNF and MANF constitute a family of neural growth factors not related to GFL. The CDNF/MANF family has been found to exhibit a neurotrophic effect on nigral dopaminergic neurons through a hitherto unknown mechanism . The factors are found in both in the extracellular environment in response to ischemia and status epilepticus as well as in the endoplasmatic reticulum where they reduce ER stress by modulating the unfolded protein response .
Realistic Competitors For Dbs
Although AADC and triple enzyme gene therapies do not represent a cure for PD, or even a slowing of disease progression, they do represent a potentially viable alternative to DBS, the current gold standard for treating motor fluctuations. As described above, the target patients in trials to date have been those with moderately advanced PD and motor fluctuations, which is exactly the group in which DBS has found its major niche.
Also Check: How Do You Get Parkinson Disease Symptoms
The Preclinical Evidence That Gdnf Can Rescue The Nigrostriatal Pathway
The discovery of GDNF in 1993 was made at a time of great interest in the therapeutic development of neurotrophic factors which offered potential for treating a number of disease states. The search for a survival factor with high selectivity for midbrain DA neurons had already been going on for some time. As such, when Lin et al. reported the cloning and bioactivity of this new trophic factor in 1993 there was great excitement . Indeed, this in part helps explain why there was such a short time span between the first pre-clinical in vivo studies and the first clinical trial with this agent which started recruiting patients in July 1996.
The initial work with GDNF was made possible through having access to a recombinant human form of protein from Synergen and Genentech. This enabled the generation of preliminary in vivo data on DA neuroprotection in the three rodent PD models available at the time: the rat 6 hydroxydopamine model , the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model and the knife-transection model . In addition, a study performed in intact rats showed that GDNF, administered into the substantia nigra, could stimulate DA neuronal function . The fact that the findings from all of these studies provided positive evidence in the same direction was re-assuring namely a growth factor that seemed to work on DA neuronal rescue and regeneration.
Strategies For Optimising Ntf Delivery In Pd

A final point on target selection is the consideration of whether intraputaminal vector treatments, such as AAV2-GDNF and CERE-120, are transported from the striatum to the substantia nigra in an anterograde or retrograde manner. Bartus et al. attributed the lack of CERE-120 transport to SNpc as failure of retrograde transport efficacy in the degenerating neurons . It was on this basis that studies involving CERE-120 infusions into both striatum and substantia nigra were initiated . However, Bankiewicz and co-workers showed that nigral cell bodies can still receive intraputaminal AAV2-GDNF via anterograde transport by nondopaminergic neurons of the striatonigral pathway . Their rationale is that targeting the nigra with NTFs carries considerable health and stereotactic risks, and that the intraputaminal CED approach alone is enough to therapeutically support the nigrostriatal neurons . One risk with efficient transfer of NTF protein by anterograde trafficking is that areas additional to the target area may receive the therapeutic protein, which may cause off-target side-effects. If the upcoming AAV2-GDNF clinical trial yields more efficacious results than the CERE-120 clinical trial , then it may be worth investigating whether CERE-120 could be more effectively delivered via intraputaminal CED. This could then be similarly applied to future viral vector-mediated delivery of other NTFs, such as CDNF and GDF5.
Read Also: Physical Therapy For Parkinson Disease Patients
What Has Been Shown With Cell
The initial trials in the early to late 1980s predominantly used adrenal medullary tissue for which the preclinical evidence was limited . The conclusion of the clinical trial work using this tissue, which was undertaken extensively across many centers especially in the USA, was that these transplants offered no real benefit and were associated with side effects and poor survival . Therefore they were abandoned, especially as more encouraging data was beginning to emerge with the use of hfVM allografts.
The move to trial hfVM in the clinic came on the back of robust reproducible preclinical findings in neurotoxic animal models of PD that had been carried out in many different laboratories around the world. These studies showed that allografts of the developing VM into rats lesioned unilaterally with 6-hydroxy-dopamine could survive, make and receive connections from the host brain, release DA, and restore behaviors to normal in these animals . This was also shown in NHP, most notably marmosets , and it was on this background that patients were first grafted in the late 1980s and thereafter till the end of the century.
As part of these discussions, a review of the data from all these trials was undertaken , and a number of conclusions were drawn, suggesting that this approach may still have merit, especially given that protocols for making human stem cell-derived DA cells of an authentic midbrain type were starting to emerge. These conclusions were that:
Gdnf Gene Therapy For Parkinsons Disease
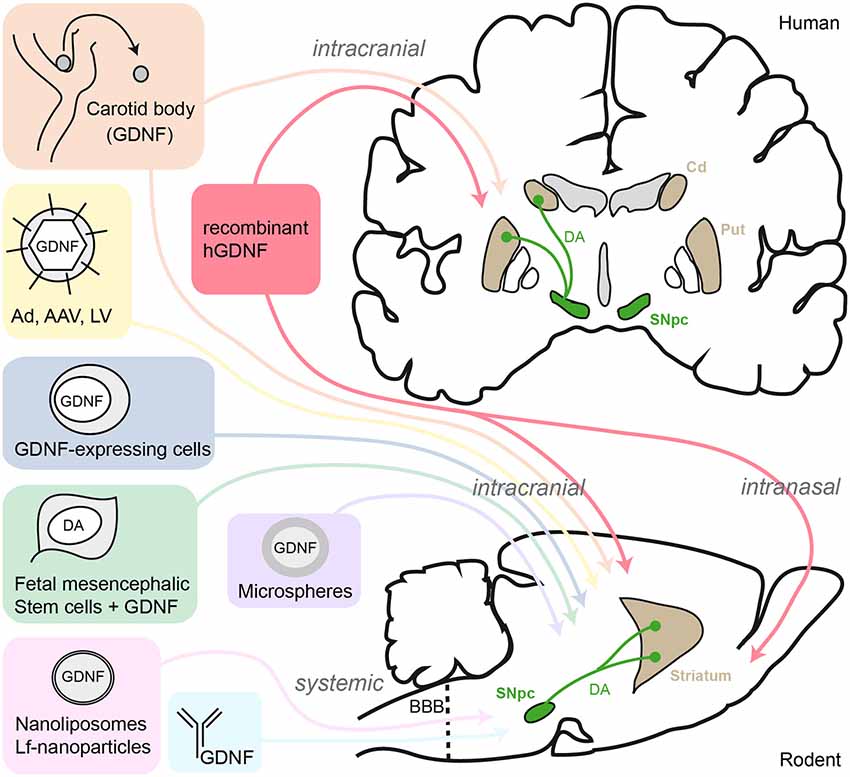
GDNF gene therapy consists of a delivery vector and a specific genetic payload. The vector is made up of the outer shell of the non-infectious adeno-associated virus serotype 2 , which provides the carrying capacity and attachment properties to specific brain cell types and transfer of its genetic payload. The genetic payload carried by AAV2 includes a specific DNA sequence coding for the GDNF protein. Within target brain nerve cells, the GDNF gene induces production and local release of GDNF protein. The genetic payload also includes regulatory sequences that provide long-term stability of the transferred GDNF gene and continuous production of GDNF protein by the recipient nerve cell.
Direct brain delivery of the AAV2-GDNF therapeutic agent is performed with intraoperative MRI guidance using customized neurosurgical methods and technologies. Real-time brain MRI during surgery provides visualization of the distribution of AAV2-GDNF and allows delivery of the therapeutic agent to be tailored to each individuals precise anatomical requirements.
Interested in clinical trials?
For more information on clinical trials for any of the diseases we have in our pipeline, all you have to do is Ask.
Don’t Miss: Are Hallucinations A Symptom Of Parkinson’s Disease
What Is The Future Of Gene Therapy For Parkinsons Disease
The jury is still out on each of the gene therapy strategies discussed above and each is still under investigation in an active clinical trial, although as of publication of this blog, none are currently recruiting participants. Three of APDAs Centers for Advanced Research are participating in gene therapy trials for Parkinsons disease. Emory University School of Medicine and the University of Alabama at Birmingham School of Medicine are participating in trials of Neurturin. The University of Pittsburgh Medical Center and Emory University School of Medicine are participating in trials of AADC.
The Early Clinical Trials
The move from the lab to the clinic is always challenging and in order to assess progress and success, new agents are often evaluated against four key elements. These include whether the drug :
- Reaches its proposed site of action at sufficient concentrations
- Shows target engagement at that site in a measurable way
- Displays functional downstream pharmacological effects
- Exhibits improvement in the relevant phenotype of the treated individuals .
The first of these criteria falls under the umbrella of delivery, the latter three provide a basis for potential efficacy, if delivery sufficient to cover the putamen can be achieved. In the sections below, we consider the open-label and double-blind, placebo-controlled clinical trials to-date in which recombinant human GDNF has been directly administered to people with PD. This will be followed by a description and discussion of the clinical studies where a related trophic factor NRTN was administered as viral vector injections to the basal ganglia as well as an ongoing GDNF gene therapy trial.
Don’t Miss: Core Exercises For Parkinson’s
Other Targets And Strategies
In addition to the above-mentioned peptides for gene therapeutic treatment of PD, several other possible genes deserve attention as potential targets in PD. One such target is the neuron restrictive silencing factor , a zinc finger transcription factor found to be involved in restriction of neuronal factors in non-neural cells and regulation of neurogenesis . Yu and associates reported that NRSF knockout mice were more susceptible to MPTP-induced dopaminergic cell death, as lack of NRSF leads to lower levels of BDNF and TH . It is possible that by upregulating NRSF in conjunction with BDNF, it would be possible to enhance the neuroprotective effects of BDNF. However, sparse data are available on NRSF in PD, but as the role of long coding RNAs have been associated with several neurodegenerative diseases, epigenetic regulation provides a novel interesting avenue of research regarding several diseases .
The transcription factor EB has been found to be a key player in regulating the autophagy-lysosomal pathway which is found to be impaired in PD . By overexpressing TFEB, Decressac et al. reported neuroprotection as well as rescue of a parkinsonian phenotype in a rat model overexpressing SNCA in the SN and ventral tegmental area . These results point to TFEB as another potential future target in the pursuit of therapies for PD.
Intranigral Vs Intrastriatal Delivery
Two delivery sites were used and sometimes combined: the SN and the striatum in order to deliver GDNF respectively to the dopaminergic neurons cell soma and at the level of their terminals. In some instances, both approaches were combined .
Most of the studies performed in the neuroprotection paradigm indicated that when GDNF was administered at the level of the SN, cell bodies were protected but no benefit on motor symptoms was observed . In contrast, when GDNF was administered into the striatum, both cell bodies and terminals were preserved and motor symptoms were reduced . Accordingly, several recent studies have suggested that axonal dysfunction precedes neuronal cell death and is better correlated with clinical symptoms .
You May Like: What Diseases Are Similar To Parkinson’s
Gene Therapy Trials With Nrtn And Gdnf
In contrast to the immense logistical challenges and potential safety concerns associated with continuous or repeated long-term delivery of recombinant GDNF protein, gene therapy promises sustained, durable and localized production of properly folded biologically active GDNF following a one-time dosing procedure. Several clinical studies have now been conducted in PD, including a multi-phase program of NRTN gene transfer, a homolog of GDNF, and more recently a Phase 1 clinical safety trial of GDNF gene transfer. Both the NRTN and GDNF gene therapy programs utilized gene transfer vectors derived from the non-pathogenic adeno-associated virus serotype 2 with a constitutive CMV promoter. These vectors appear to have a favourable safety profile for neurotrophic factor gene delivery in PD, in addition to which AAV2 has an exclusive neuronal tropism and restricted distribution when directly delivered to the brain , thus minimising off target side effects.
Several key changes were made as part of the AAV2-GDNF Phase 1 study design compared to prior direct infusion studies in PD conducted in the early 2000s, including:
Quantification Of Cell Numbers
Quantification of SNpc cell numbers has been described in detail elsewhere . Briefly, three coronal sections were used to quantify TH, VMAT2, pRPS6 positive SNpc neurons: the coronal section containing medial lemniscus separating ventral tegmental area from SNpc , adjacent cranial section, and adjacent caudal section. Data were presented as a percentage of neurons relative to the left intact side.
Read Also: Signs Of Parkinson Disease Early Symptoms
Potential Of Dopaminergic Ntfs For Treatment Of Pd
There is a pressing need for disease-modifying therapies for PD, since currently-used treatments focus on symptom management rather than on their cause. NTFs hold significant promise in this respect. The most widely-used pharmacological approach is administration of levodopa , a precursor of dopamine, to replenish diminished levels of striatal dopamine. However, at least half of L-DOPA users experience drug-induced dyskinesia or other motor complications after about 5 years of therapy. Agents which are used in combination with L-DOPA to optimise its effects include DA agonists, peripheral aromatic amino acid decarboxylase inhibitors, monoamine oxidase B inhibitors and catechol-O-methyl transferase inhibitors . Alternatives to pharmacological treatment, for patients in which L-DOPA therapy does not work well or no longer works, include deep brain stimulation . DBS confers improvements in motor symptoms due to ablation of brain structures such as the thalamus or pallidum. This strategy is not without complications and is typically used as a last resort to manage motor symptoms . Like L-DOPA, DBS does not halt or slow the progression of the neurodegeneration. Thus, other approaches, including cell-based therapies and NTFs, are under intensive investigation as disease-modifying therapies.
Cell Type Secreting Gdnf
In the striatum, endogenous GDNF is expressed by parvalbumin-positive interneurons . The different classes of viral vectors used to deliver GDNF transduce different cell types with varying efficiencies. The promoter used for transgene expression further influences the cell type specificity . In most studies, this issue has not been evaluated. Some vectors transduce the more abundant medium spiny neurons which project to the globus pallidus and SNr, thus resulting in an anterograde transport of the transgene product in these structures . In order to avoid this dissemination of GDNF in non-targeted structures which can provoke undesired effects , some groups have directed GDNF expression into astrocytes in order to restrict transgene expression to the delivery site in the striatum . Considering the different cell type specificities of transgene expression mediated by the different vectors and the variable amounts of GDNF produced , it is very difficult to compare studies performed using different viral vectors.
Recommended Reading: What Causes Parkinson’s Syndrome
Askbio Adds Cns Gene Therapy Programs In Parkinsons Disease And Multiple System Atrophy
Clinical team led by neurological gene therapy expert, Dr. Krystof Bankiewicz, joins AskBio
Research Triangle Park, N.C. January 28, 2021 Asklepios BioPharmaceutical, Inc. , a clinical-stage adeno-associated virus gene therapy company and wholly owned subsidiary of Bayer AG, today announced that the clinical team from Brain Neurotherapy Bio, Inc., a gene therapy company based in Columbus, Ohio, has joined AskBio to expand its clinical pipeline for the treatment of neurodegenerative disorders. Brain Neurotherapy Bio was established in 2018 by Krystof Bankiewicz, MD, PhD, in association with AskBio founders Sheila Mikhail, JD, and Jude Samulski, PhD. Dr. Bankiewicz will lead AskBios development efforts in Parkinsons disease and multiple system atrophy .
Im pleased to welcome the Brain Neurotherapy Bio team to AskBio, said Sheila Mikhail, AskBio CEO. They bring two active clinical programs, proprietary delivery technologies and unique clinical expertise in CNS indications, including the clinical translation and development of neurotrophic factor gene therapy for the treatment of neurological conditions.
Dr. Bankiewicz commented, We are grateful for the collaboration weve had with AskBio and are honored to jointly accelerate clinical development of our gene therapy programs that we hope will benefit patients and families living with Parkinsons disease, multiple system atrophy and other neurological conditions.
Neurotrophic Factors Gene Delivery: Still A Promising Clinical Paradigm For Parkinson’s Disease
Clinical trials were conducted using catheters releasing recombinant GDNF protein as well as rAAV serotype 2 -mediated delivery of the NRTN or GDNF cDNA . In the Phase I trials, AAV vectors were safe. However, the Phase II results were disappointing, although beneficial effects have been described for patients which have been followed for longer periods . Several factors could have reduced clinical benefits.
Read Also: Parkinson’s Staring Into Space
Gene Therapy To Synthesize Dopamine
Within the basal ganglia, DA is delivered to the striatum by axonal projections from the substantia nigra , which thus regulate striatal function and motor and cognitive performance. In the striatum, the main population of striatal output neuronsmedium spiny neurons are exposed to DA in both tonic and phasic modes, with phasic release being tightly correlated with circuit activity and behavior, and important for habit learning . Phasic DA responses cannot be reconstructed by gene delivery to the striatum, but tonic DA exposure seems sufficient to ameliorate many of the features of PD in both patients and animal models. Replenishment of DA supply through GT is therefore an obvious approach. To date, two vector/cargo combinations have been employed to achieve resupply of DA to the DA-denervated putamen. The aim of this therapy is illustrated in Fig. 1D, and the relevant clinical trials are summarized in Table 2.
Table 2 Summary of dopamine synthetic GT trials to date
Avoid Undesirable Compensatory Effects Of Sustained Gdnf Administration At Supraphysiological Doses
Except in one study , in which GDNF striatal concentration was only three-fold higher than the endogenous level, in most preclinical studies it was increased at least 10-fold . In such conditions of excessive GDNF overexpression, time-dependent compensatory mechanisms affecting both the motor behavior and DA biosynthesis and turn-over were observed . Therefore, GDNF administration should be adjusted to a concentration which does not perturb DA homeostasis and the treatment should be interrupted before the appearance of compensatory effects.
Recommended Reading: Alcohol And Parkinson’s Disease