Inclusion And Exclusion Criteria
The studies were selected based on the following criteria: randomized controlled trials involving patients with advanced PD receiving levodopa each article must contain at least one outcome variable, such as the number of adverse events, number of dyskinesia symptoms, change in total ON-time, Unified PD Rating Scale part III scores, and total daily dose of levodopa each article must include at least one adjuvant drug for the treatment of PD, including opicapone, entacapone, and tolcapone all subjects must be Parkinson’s patients with fluctuating symptoms.
The publications were excluded according to the following criteria: incomplete data or lack of statistical analysis reviews, comments, and letters duplicate articles or multiple surveys based on the same data and 4) open-label studies
Increasing Adoption Of Oral Route Of Administration Will Foster The Market Size
Oral segment in the Parkinsons disease therapeutics market exceeded USD 3.41 billion in 2020 impelled by the factors such as pain avoidance, ease of ingestion, patient compliance and versatility to accommodate various types of drugs. Several dopamine agonists including Ropinirole and Pramipexole are administered through oral route of administration for increased clinical outcome. Thus, these factors are growing the preference for oral route of administration that will in turn propel the overall industry growth.
Get more details on this report – Request Free Sample PDF
Parkinsons disease therapeutics market for Adult patient segment accounted for USD 4.35 billion in 2020 propelled by the increasing prevalence of Parkinsons disease in adults. According to a published article, in the U.S., in 2017, approximately 90,000 commercially insured adults between the age of 30 and 64 years were diagnosed with Parkinsons disease. In addition, men are 1.5 times more likely to suffer from Parkinsons disease than women.
Get more details on this report – Request Free Sample PDF
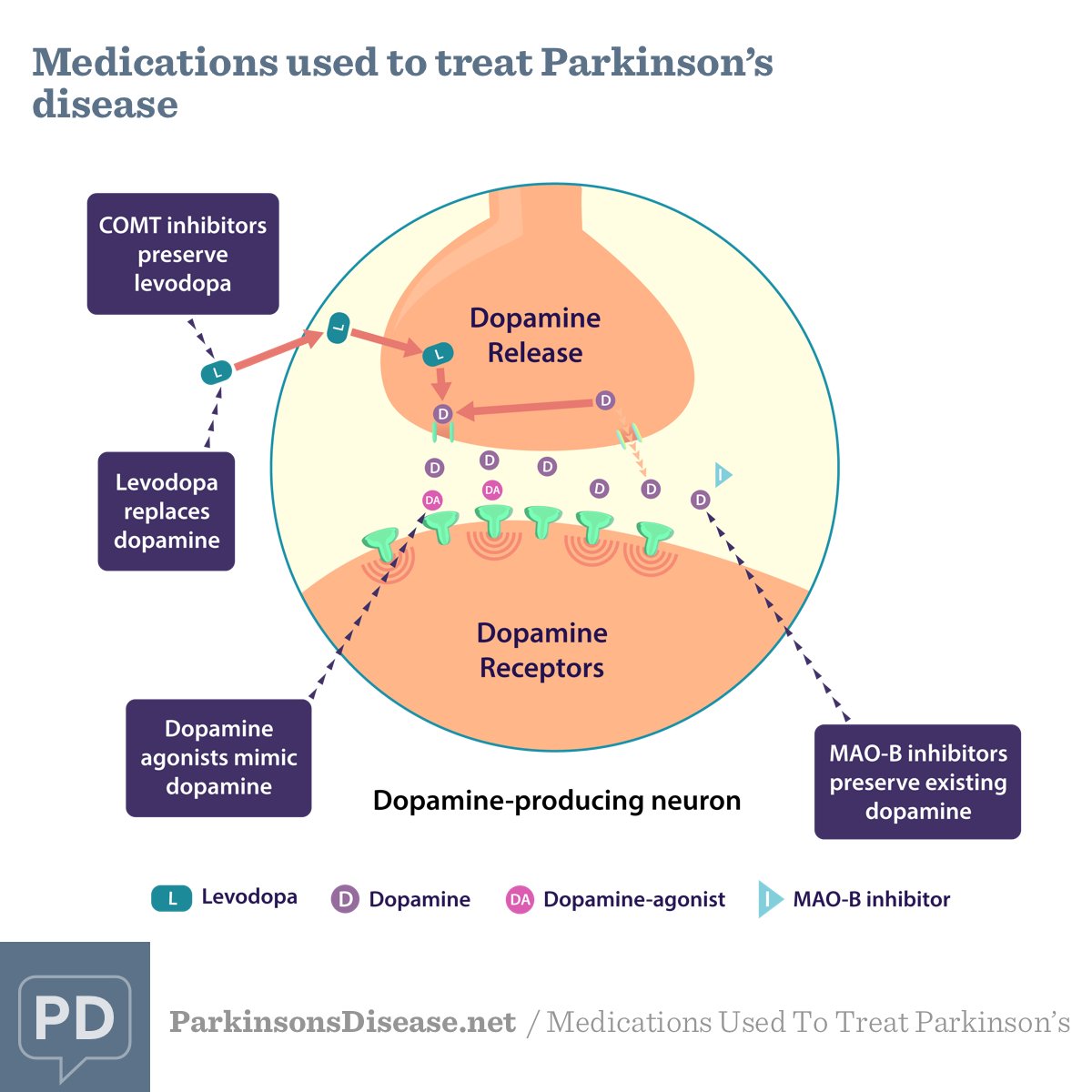
Types Of Comt Inhibitors For Parkinsons
Comtan and Tasmar are two COMT inhibitors that doctors use to treat Parkinsons disease. Tasmar is more potent than Comtan, but may cause liver damage.
The European Commission approved a new once-daily oral COMT inhibitor, Ongentys , in 2016. The U.S. Food and Drug Administration also approved Ongentys in April 2020 after two successful Phase 3 clinical trials .
Ongentys has a very high binding affinity to the COMT enzyme, which results in a long duration of action. This allows physicians to lower the dose of levodopa further and reduce off episodes.
You May Like: Parkinson’s Leaning To One Side
Comt Inhibitor Drugs: An Introduction
Levodopa can boost the supply of dopamine in your brain. COMT inhibitors can block an enzyme that breaks down levodopa medication. This helps it to work more effectively.
COMT inhibitors do not help to manage the symptoms of Parkinsons on their own they have to be used with levodopa.
Your specialist might prescribe them if your dose of levodopa is not working for long enough.
It will also help your levodopa medication to work more smoothly in controlling your symptoms.
Quality Assessment And Data Extraction
The Cochrane Collaboration risk of bias assessment tool was used to evaluate the quality of all selected articles . After extracting and identifying eligible articles, two reviewers extracted relevant data for independent evaluation, including data on first author, publication year, study area, follow-up time, total number of included participants, population age, and sex ratio. In addition, if there were any disagreements during data extraction and quality assessment, a conclusion was reached after discussion with a third reviewer.
You May Like: Parkinson’s And Physical Therapy
Treatment Of Neurobehavioral Features
Treatment of cognitive deficits associated with PD is as challenging as the treatment of Alzheimers disease and other dementias. While the general assumption has been that cognitive deficits are a feature of late-stage PD, clinically inapparent cognitive changes on neuropsychiatric testing may be found . With the introduction of cholinesterase inhibitors such as donepezil , rivastigmine , and galantamine and the NDMA antagonist memantine , it is possible that cognition, orientation and language function will improve, and that such improvement will lead to a meaningful improvement in function. Both donepezil and rivastigmine improve cognition to the same effect, but donepezil is better tolerated . The largest and best-designed study of rivastigmine in dementia associated with PD involved 541 patients enrolled in a 24-week randomized, multicenter, double-blind clinical trial . The patients had a relatively mild dementia , with onset of dementia about 2 years after onset of PD symptoms. The mean ADAS-cog score, the primary efficacy variable, improved by 2.1 points in the rivastigmine group, compared to 0.7 in the placebo group , and the MMSE improved by 0.8 in the rivastigmine group and worsened by 0.2 in the placebo group . At the end of the study, 55.5% were receiving 9 to 12 mg. The adverse effects that were significantly more frequent in the rivastigmine group were nausea, vomiting, dizziness, and tremor.
Impulsive And Compulsive Behaviours
Behaviours may involve gambling, becoming a shopaholic, binge eating or focusing on sexual feelings and thoughts. This can have a huge impact on peoples lives including family and friends.
Not everyone who takes Parkinsons medication will experience impulsive and compulsive behaviours, so these side effects should not put you off taking your medication to control your symptoms.
Find out more about impulsive and compulsive behaviours.
Don’t Miss: Parkinson’s And Stem Cell Treatment
Examples Of Comt Inhibitors
The 3 COMT inhibitors used in the treatment of PD are:1
- Comtan®
- Diarrhea
- Urine discoloration
Tolcapone may cause potentially fatal liver failure. Because of this serious side effect, it should only be considered for people who are not getting enough symptom control or candidates for other therapies.1,5
These are not all the possible side effects of COMT inhibitors. Talk to your doctor about what to expect or if you experience any changes that concern you during treatment with COMT inhibitors.
Quality Assessments Of The Selected Literature
The quality evaluation of randomized controlled trials revealed that the overall quality of publications we included was relatively high as exhibited in Figure 2. Despite the assessment results of many articles show unclear in terms of selective reporting indicator, there were only two literatures having higher risks in terms of other bias which was caused by insufficient follow-up of 6 weeks.
Figure 2. Risks of bias assessment.
Also Check: Ptsd And Parkinson’s Disease
Growing Preference For Catechol
COMT inhibitors segment is predicted to witness 8.8% growth rate till 2027 led by the high efficacy of Catechol-O-methyltransferase inhibitors in alleviating the motor symptoms of Parkinsons disease. COMT inhibitors have proven to be beneficial adjunct to levodopa therapy as they increased the bioavailability of LD and prolong the elimination of LD in Parkinsons disease patients. The advantages associated with COMT inhibitors are essential to augment their utilization and demand.
Role Of Comt In Parkinson
COMT inhibitors play an important role in modifying the pharmacokinetics of levodopa, stated Werner Poewe, MD, the director of the Department of Neurology at the Medical University of Innsbruck in Austria, at the 2016 German Society of Neurology Congress in Mannheim, Germany.4
COMT is an enzyme that eliminates levodopa by catalyzing the conversion of levodopa to 3-O-methyldopa, a metabolite that has no known therapeutic value. COMT inhibitors block this metabolic pathway in the periphery, thereby increasing the amount of levodopa that is available to be converted into dopamine .
As a result of this process, COMT inhibitors enhance the therapeutic effect of levodopa by increasing its bioavailability and sustainability, thus preventing wearing off and improving clinical responsiveness.5 Importantly, COMT inhibitors have no direct effect on Parkinson symptoms, so they are effective only when administered in combination with levodopa.
Don’t Miss: Caring For Someone With Parkinson’s Disease
Pharmacokinetics Of Comt Inhibitors
Following oral administration, tolcapone, entacapone, and nebicapone are rapidly absorbed, but due to significant first-pass metabolism their oral bioavailability is not complete. The oral bioavailability ranges between approximately 35% for entacapone and 60% for tolcapone . The maximum plasma concentration is reached within 0.5â2 h their elimination is rapid, with apparent elimination half-life between 1.6 and 3.4 h. The main pharmacokinetic parameters following single-dose oral administration in healthy subjects are summarized in .
Main pharmacokinetic parameters following single oral doses of COMT inhibitors in healthy subjects
| Compound and oral dose | |
|---|---|
| â16.0* | 2.0 |
- *AUC0-tâ Area under the plasma concentration-time curve from time 0 to the last sampling time at which concentrations are equal or above the limit of quantification.
- AUCââ AUC from time 0 to infinity Cmaxâ maximum plasma concentration t½βâplasma apparent terminal elimination half-life Tmaxâ time to reach Cmax.
Area under the plasma-concentration time curve and Cmax values are dose-proportional after tolcapone , entacapone , and nebicapone oral administration . The volume of distribution is relatively small and all nitrocathecols are highly bound to plasma proteins . The pharmacokinetics of COMT inhibitors is not significantly influenced by the presence of food.
Comt Inhibitors And Mao
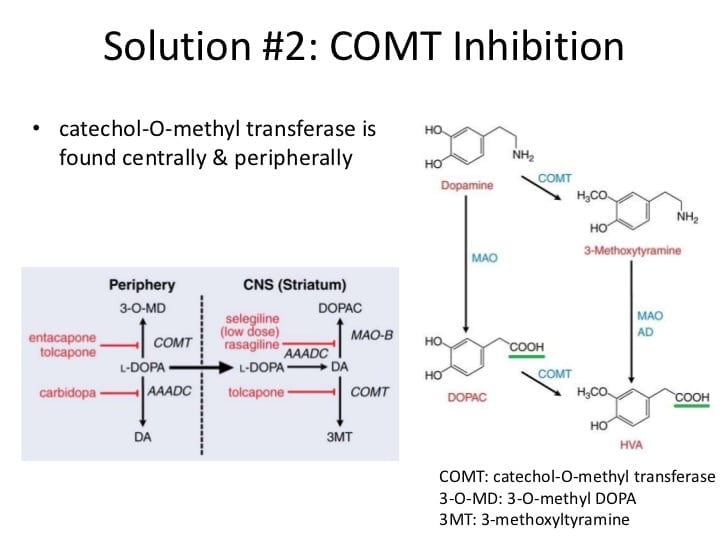
Catechol O-methyltransferase is an enzyme that breaks down Levodopa into a useless form that eventually gets eliminated from the body. To treat the motor symptoms of Parkinsons disease, Levodopa is prescribed as a pill that will eventually be converted into dopamine in the brain to restore the stores that have been depleted as the dopamine producing neurons die. COMT will alter L-dopa by adding a methyl group, which effectively prevents it from ever becoming dopamine. COMT can also be utilized in combination with monoamine oxidase , another enzyme, to degrade dopamine through a different pathway. There are two forms of MAO: A form and B form. The B form is most commonly targeted in PD treatment because it is especially active in breaking dopamine down into a useless form.
In healthy individuals, these methods are important to maintaining a normal concentration of dopamine in the brain because they are important for eliminating dopamine once used. However, in individuals with Parkinsons disease, these pathways are functioning without a system to produce dopamine, so any action by these degradation pathways is harmful.
In order to treat PD, medications have been developed to prevent COMT and MAO from breaking down dopamine or its precursors. These medications inhibit that actions of COMT and MAO-B so the overall concentration of dopamine in the brain can be increased.
Also Check: Parkinson’s And Eye Twitching
Clinical Efficacy Of Comt Inhibitors
Tolcapone, entacapone, and nebicapone increase levodopa bioavailability in PD patients, similar to what occurs in healthy volunteers, by significantly increasing levodopa AUC, leaving Cmax and Tmax without significant changes. A more sustained and less fluctuating levodopa level is obtained, which results in an improved therapeutic response and lower risk of developing dyskinesias . Patients experience an increase in the daily ON time and correspondent reduction of OFF time, with consequent improvement in the activities of daily living and general quality of life . In patients with nonfluctuating Parkinson’s disease the administration of COMT inhibitors may also have beneficial effects in daily living activities and motor functions as was observed with tolcapone .
Heterogeneity And Consistency Analysis
To assess the heterogeneity among the included studies, we performed heterogeneity analysis on each indicator we chose which compared the difference between direct and indirect comparisons. The pairwise I2 values of different indicators was show in Figures 5AE. According to I2 values of overall networks, we chose to use random effect model to perform meta-analysis in networks of change in total ON-time , total daily dose of levodopa and change in UPDRS part III scores . The other two indicators, the number of dyskinesia symptom and any adverse events , were meta-analyzed by fixed effect model as was stated in methods. To find out the consistency of three networks containing indirect comparisons, we used the node-splitting model to test the differences between direct and indirect comparisons. The goal was to determine the consistency between direct and indirect evidences of a particular node . We also found no obvious inconsistencies in the network model with indirect sources which results were shown in Figures 5FJ. Therefore, the results of the consistency model were reliable. In addition, the potential scale reduction factor value of all parameters was limited to 1, showing good convergence and effectiveness.
You May Like: Best Food For Parkinson Patient
Medical Treatment Of Parkinsons Disease
Enormous progress has been made in the treatment of Parkinsons disease over the past half century, but levodopa remains the most potent drug for controlling PD symptoms . Prior to instituting medical therapy, a correct diagnosis of PD must be established and the level of impairment determined . Each patients therapy is to be individualized, and diverse drugs other than levodopa are presently available. Among these are the dopamine agonists , catechol-o-methyl-transferase inhibitors and nondopaminergic agents . Head-to-head comparisons of drugs within classes are rare, and the differences that have emerged are related to the effects on motor fluctuations, dyskinesias, on/off times and adverse effects of the specific agents within each class .
Effect On Levodopa Pharmacokinetics
COMT inhibitors have a significant effect on the systemic availability and elimination of levodopa. In healthy volunteers, the combined administration of levodopa/AADC inhibitor with increasing doses of entacapone, tolcapone, or nebicapone, 50, 100, and 200 mg, leads to a dose-dependent increase in levodopa AUC without significant changes in levodopa Cmax or Tmax. Doses of COMT inhibitors above 200 mg may not increase levodopa systemic availability . Explanations that have been considered include degradation of levodopa by other metabolic pathways, while inhibition of COMT becomes more marked , or competition during absorption between the COMT inhibitors and levodopa for the saturable transport of large neutral amino acids at the proximal small intestine level . This competition for absorption between the COMT inhibitors and levodopa may also explain the trend for an increase in mean levodopa Tmax values when the dose of COMT inhibitor increases, as has been reported for all COMT inhibitors .
The formation of 3-OMD from levodopa is dependent on COMT activity, particularly at the intestinal level, the main site of O-methylation of levodopa. For all the COMT inhibitors, dose-dependent decreases of 3-OMD Cmax and AUC values were reported. For a dose of 200 mg, the magnitude of 3-OMD decrease was 65% for tolcapone , 45% for entacapone , and 61% for nebicapone .
Read Also: Communicating With Parkinson’s Patients
Insufficient Data Are Available On The Benefits Of The Comt Inhibitor Tolcapone Compared With The Dopamine Agonists Bromocriptine And Pergolide In Relieving The Symptoms Of Later Parkinson’s Disease
As Parkinson’s disease progresses the control of the symptoms often requires the addition of other drugs to levodopa. The principle aim of COMT inhibitor therapy is to increase the duration of effect of each levodopa dose and thus reduce the time patients spend in the relatively immobile ‘off’ phase. However other drugs such as dopamine agonists can also be used at this stage of the disease. This review found that the COMT inhibitor tolcapone as an adjuvant to levodopa treatment had a similar level of benefits as two dopamine agonists, bromocriptine and pergolide. There was no significant difference in efficacy between the adjuvant tolcapone and adjuvant bromocriptine or pergolide in the medium-term. Tolcapone produced nausea less often than these agonists but there was some evidence of liver function abnormalities with tolcapone. Post-marketing surveillance identified three cases of fatal hepatic toxicity in patients treated with tolcapone. As a result, tolcapone has been withdrawn from some countries and severe restrictions on its use have been imposed in others.
No evidence was found comparing entacapone with other adjuvant drugs for Parkinson’s disease.
As Parkinson’s disease progresses the control of the symptoms often requires the addition of other drugs to levodopa. The principle aim of COMT inhibitor therapy is to increase the duration of effect of the levodopa dose and thus reduce the time patients spend in the relatively immobile ‘off’ phase.
Pharmacodynamics Of Comt Inhibitors
In clinical trials, the COMT inhibitory effect is usually evaluated by assaying the erythrocyte S-COMT activity. All COMT inhibitors decrease erythrocyte S-COMT activity in a dose-dependent and reversible fashion. The time to maximum inhibition is rapid , but the level of inhibition and the time for enzyme activity recovery may differ with the inhibitor. The COMT inhibition profile is similar for tolcapone and nebicapone, and both these inhibitors cause a more profound and sustained inhibition than entacapone. Following oral doses of 100 mg and 200 mg, maximum COMT inhibition is, respectively, 72% and 80% for tolcapone and, respectively, 69% and 80% for nebicapone . COMT activity returns to the baseline at approximately 18 h following administration of a 200-mg dose of either tolcapone or nebicapone . With a 200-mg dose of entacapone, maximum COMT inhibition is 65% and the enzyme recovers full activity within 8 h . In a comparative study in patients with Parkinson’s disease, 75 mg of nebicapone and 200 mg of entacapone caused a similar peak COMT inhibition , but the inhibitory effect of nebicapone, 75 mg, was more sustained than that observed with entacapone, 200 mg .
Don’t Miss: Parkinson’s Foundation Kansas City
Comt Inhibitors In Development
Opicapone is a novel, once-daily COMT inhibitor that was approved for use in Europe in 2016 based on the results of 2 clinical trials, BIPARKI and BIPARKII.22 It is currently under development for use in the United States.
The safety and efficacy of opicapone were established in the afore& shy mentioned randomized, double-blind, placebo-controlled phase 3 clinical trials.23-25 In the BIPARKI trial, opicapone was compared with placebo and entacapone in 600 patients with Parkinson disease. Opicapone was found to be superior to placebo and equivalent to enta& shy capone at reducing time in the off state. Specifically, it was estimated that opicapone reduced off episodes by an average of 60 minutes.
Compared with the estimated 40-minute reduction with entaca& shy pone, this suggests that opicapone may have a greater magnitude of the effect. Importantly, these results were found for a once-daily dose of opicapone, establishing it as the first-ever COMT inhibitor that allows once-daily dosing.23,24
The BIPARKII trial validated that once-daily opica& shy pone is well tolerated and more effective at reducing end-of-dose wearing off than placebo.24 Further, both trials demonstrated that the effects of opica& shy pone were maintained for 1 year, during which the levodopa dose was not increased.
The most common AEs associated with opica& shy pone treatment included dyskinesia, constipa& shy tion, and dry mouth.