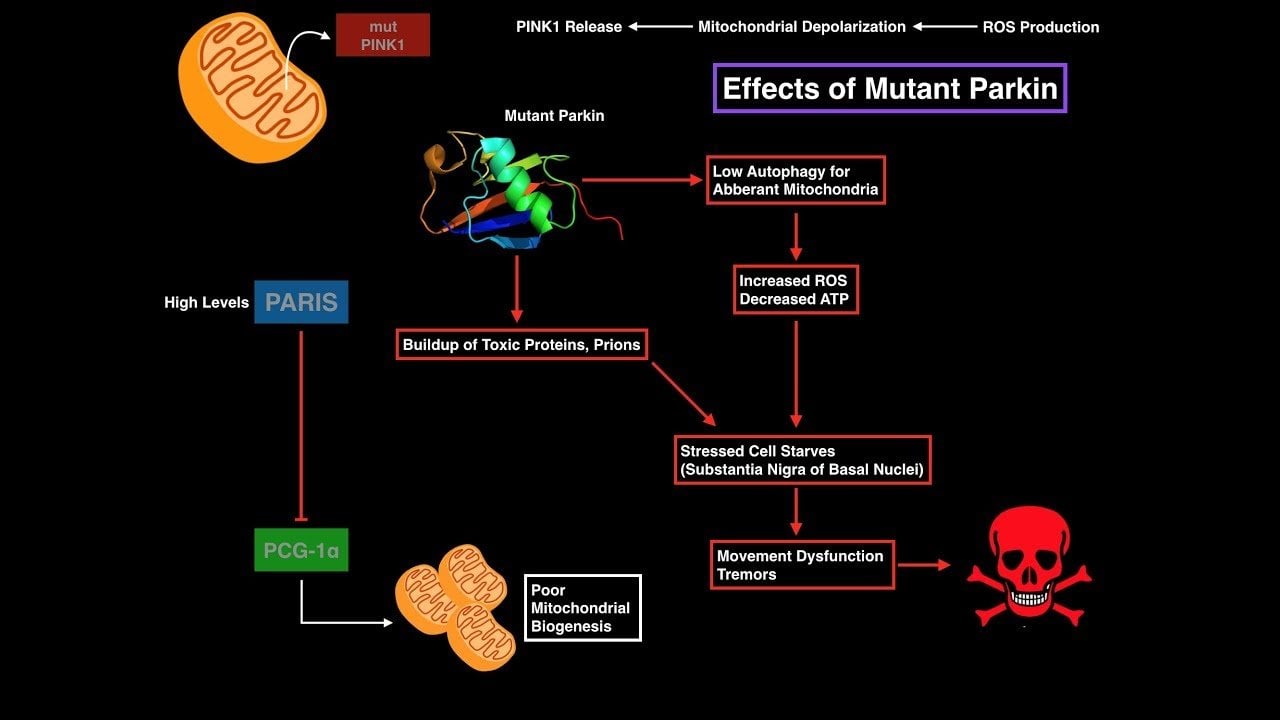
Is Parkinsons Disease A Prion Disorder
SAN DIEGOAn increasing body of evidence suggests that Parkinsons disease is a prion disorder, according to a review presented at the 65th Annual Meeting of the American Academy of Neurology. If the prion hypothesis is confirmed, it could lead to the pursuit of novel therapeutic targets.
Developing new medical treatments will still require an enormous amount of work, said C. Warren Olanow, MD, Professor of Neurology and Neuroscience at the Mount Sinai School of Medicine in New York City. Many compounds will have to be tested in high-throughput studies to find lead compounds that interfere with the prion process, but this approach currently offers the best hope for finding a cure for Parkinsons disease.
Alpha Synuclein May Be a Prion
A prion is an infectious agent made solely of misfolded protein. The most common illness caused by prions in humans is CreutzfeldtJakob disease.
The significance of the Lewy body is not known, but it may represent a protective mechanism that forms in an attempt to clear and segregate misfolded protein aggregates, said Dr. Olanow. Furthermore, recent evidence demonstrates that misfolded alpha synuclein protein filaments and aggregates can transfer to unaffected cells to extend the disease process.
Research Has Supported the Prion Hypothesis
Prion Hypothesis Could Yield Novel Therapeutic Targets
Implications For Therapeutic Strategies
For the treatment of PD, the most effective therapy to inhibit the propagation of -syn is knock-out of the related gene or inhibiting protein expression via gene or RNAi therapy. However, as mentioned earlier, it is still unknown whether gene therapy will affect -syn normal function. Further studies are needed to evaluate the relevant efficacy before the approach can be used for clinical treatment .
Furthermore, immunotherapy targeting extracellular -syn is showing great promise. Experiments in PD models indicate that active vaccination against recombinant -syn leads to ameliorated pathology . Similarly, passive immunotherapy also produced positive effects. Antibodies produced by immunotherapy are expected to block the spreading of pathological -syn . Moreover, the spreading of -syn in wild-type mice after intracerebral inoculation of -syn fibrils was prevented by monoclonal antibodies against abnormal -syn. -Syn mAbs reduce -syn PFF-induced LBs/LNs formation and rescue synapse/neuron loss in primary neuronal cultures by preventing both uptake and subsequent cell-to-cell transmission of pathology .
Several diagnostic imaging probes were reported to monitor cerebral amyloid lesions in neurodegenerative disorders. Florbetapir is the first radioactive dye for brain imaging of amyloid plaques approved by the FDA in AD. The importance of further studies for their practical implications in therapy and diagnostics should be highlighted .
Alzheimers Disease Is A Double
Self-Propagating Amyloid and Tau Prions found in Post-Mortem Brain Samples, With Highest Levels in Patients Who Died Young
The normal form of Aß has been tagged with a yellow marker in these cells, making healthy cells a uniform pale yellow . Contact with prion forms of Aß for example in extracts from human brain tissue forces these yellow proteins into the sticky prion form as well, leading to the formation of bright yellow clumps .
Two proteins central to the pathology of Alzheimers disease act as prions misshapen proteins that spread through tissue like an infection by forcing normal proteins to adopt the same misfolded shape according to new UC San Francisco research.
Using novel laboratory tests, the researchers were able to detect and measure specific, self-propagating prion forms of the proteins amyloid beta and tau in postmortem brain tissue of 75 Alzheimers patients. In a striking finding, higher levels of these prions in human brain samples were strongly associated with early-onset forms of the disease and younger age at death.
Senior author Stanley B. Prusiner, MD, director of the UCSF Institute for Neurodegenerative Diseases and professor in the departments of Neurology and of Biochemistry and Biophysics. Image courtesy UCSF Institute for Neurodegenerative Diseases.
You May Like: Does Vitamin B12 Help Parkinson’s
Prion Diseases And Prion
Prion diseases are fatal transmissible neurodegenerative disorders with genetic, sporadic, and acquired forms . Prions are the infectious agents responsible for the transmissible spongiform encephalopathies , a group of lethal neurodegenerative diseases . These diseases also include Kuru, Creutzfeldt-Jakob disease , GerstmannSträusslerScheinker disease , and fatal familial insomnia in human, bovine spongiform encephalopathy in cattle, and scrapie in sheep .
Figure 1. The Templated Conformation Change of Prions. Step 1- The -helices of PrPC transform into the -sheets conformation to form pathological PrPSc. Step 2- PrPSc interacts with PrPC and converts them into pathological form. Step 3- PrPSc binds to the cognate prion molecules. Unstable oligomeric species grow by recruiting additional unfolded or oligomeric species of the same protein until forming a stable nucleus. Step 4- The prion aggregates break into small fragments that act as seeds and spread indefinitely from the point of infection to the CNS.
Emerging research highlights that many neurodegenerative diseases share key prion-like similarities with the progress of prion diseases. -Syn is a typical pathogenic agent for PD and exhibits properties of self-aggregation and propagation, just like prions do.
Parkinsons Disease Usually Begins Between The Ages Of 50 And 65 It Is Slightly More Common In Men Than Women
The protein alpha-synuclein plays a critical role in the pathogenesis of Parkinsons disease. Alpha-synuclein accumulates in the brains of patients with Parkinsons disease. It starts in a region of the lower brain called medulla oblongata from where it spreads upwardly toward midbrain and cortical areas.
Like prion diseases, misfolded alpha-synuclein and tau protein associated with Parkinsons disease and Alzheimers disease, respectively, have been reported in skin tissues of patients with these conditions. Skin could serve as a screen for early diagnosis, but also for monitoring the accumulation of the misfolded proteins in the brain of these neurodegenerative diseases. It also could be further confirmation that all cell tissue and bodily fluids are infectious.
The basal ganglia area of the brain regulates body movements. Its cells require a proper balance of dopamine and acetylcholine, both involved in the transmission of nerve impulses. In Parkinsons, cells that produce dopamine begin to degenerate, which upsets the balance of these neurotransmitters.
Michael J. Fox has bravely become the most famous spokesperson for victims of the disease. His work has generated a great deal of resources for research.
Copyright
Read Also: Parkinson Bicycle Cleveland Clinic
Why Do Not All Synucleinopathies Look And Behave The Same
The aggregation of nonmutant -SYN can cause distinct synucleinopathies in humans. The fact that -SYN can form fibrillar assemblies with distinct structural characteristics, as discussed above, has led to the hypothesis that -SYN strains may account for the different clinicopathological traits that characterize DLB, MSA, and PD. Indeed, -SYN molecules adopt different conformations leading to distinct molecular stacking within distinct fibrillar assemblies and surfaces dissimilarities. As explained above, in addition to having different biophysical properties that reflect into different seeding, persistence, and macroscopic appearance, those assemblies have surface characteristics that likely account for distinct interactomes. Thus, by interacting with different partners, including membranous surface proteins, diverse -SYN strains perturb cellular proteostasis in different ways leading to strain-specific clinicopathophysiological traits. This is a potential explanation for why -SYN aggregates mostly appear in oligodendrocytes in MSA and in neurons in PD/DLB. Patients with DLB, PD, and PD with dementia also exhibit different initial clinical symptoms and diverse rates of progression. While this potentially is also influenced by the predominant strain of -SYN, another explanation might be that the primary initial trigger site of LP is different .
Related Work And Trials
Since this review was published, further research has highlighted the prion-like mechanism as important for the spread of alpha-synucelin from cell to cell in Parkinsons. Recent research emphasizes the time lag between the formation of protein clumps in cells and appearance of the first clinical symptoms as a prodromal phase of Parkinsons meaning that alpha-synuclein clumping and seeding in this manner can be a marker of Parkinsons before the presentation of any symptoms.
Recommended Reading: On-off Phenomenon
Who Is J Bart Classen
It turns out that hes been a subject on this particular blog before, primarily as scientist who gave antivaxxer Robert F. Kennedy, Jr. ammunition for his false claim that vaccines are responsible for the obesity epidemic. According to Wikipedia, Dr. Classen received his MD from the University of Maryland, Baltimore in 1988 and also has an MBA from Columbia University. Unsurprisingly, hes been quoted by Sharyl Attkisson, a reporter whos become an antivaccine activist and conspiracy theorist in her own right. Classens website, Vaccines.net, is pretty rudimentary but does proclaim:
The content of this site is not intended to be anti-immunization but instead to promote the concept that the goal of immunization is to promote health not eradicate infections. It is hoped that through the collection and dissemination of information about the chronic effects of vaccines, safer immunization practices will become available for those who choose to be immunized.
There it is, the Im not antivaccine Im a pro-safe vaccine gambit, beloved of antivaxxers going back at least to Jenny McCarthy 14 years ago. Naturally, after proclaiming himself a vaccine safety advocate, Dr. Classen then goes on to spout antivaccine misinformation:
And now he thinks that mRNA-based COVID-19 vaccines can cause prion disease leading to neurodegenerative diseases like Alzheimers disease. With zero evidence to support his idea and very close to zero biological plausibility. Of course.
What Are The Initial Triggers Of
Assuming that cell-to-cell transfer of -SYN assemblies plays an important role in PD pathogenesis, two important follow-up questions are how and why does this process start? In this section, we discuss these questions.
One can view misfolding and aggregation of -SYN as a stochastic event that occurs throughout life. Under some cell stress conditions , which we discuss further below, -SYN misfolding is promoted. Normally, neurons clear this garbage , but we suggest that on rare occasions the proteostasis mechanisms fail and then the pathogenic process starts .
As stated above, the thermodynamic stability of such intermolecular interactions depends on the concentration of the assembly competent conformers . Point mutations within the -SYN encoding gene, SNCA, increase or decrease the number of possible conformations -SYN adopts, and affect the lifespan and cellular concentration of these conformations. Duplication and triplication of SNCA also affect the lifespan and concentration of assembly competent conformers. This is why certain point mutations and gene duplication/triplication are associated with increased aggregation propensity and early onset of PD-like conditions . Furthermore, single nucleotide polymorphisms in a distal SNCA enhancer are associated with altered PD risk , and experiments in neurons differentiated from induced pluripotent stem cells suggest that very modest changes in -SYN expression significantly impact life-time PD .
Read Also: Similar To Parkinsons
Braak Staging Of Parkinsons Disease
Braak and colleagues devised their widely accepted staging system of PD progression by assessing the regional distribution of syn immunoreactive structures in the brains of 110 syn positive subjects . According to the Braak model, the disease process commences in the lower brainstem in the dorsal motor nucleus of the vagus nerve , as well as anterior olfactory structures. The disease then ascends rostrally from the DMV through susceptible regions of the medulla, pontine tegmentum, midbrain, and basal forebrain, eventually reaching the cerebral cortex . This is a non-random, progressive process, with specific nuclei and neuronal types giving rise to the development of Lewy pathology in a stereotypic pattern. As the pathology advances upwards from the brainstem, both the severity of the lesions and the clinical manifestations of the disease increase .
Figure 1
Variations On A Theme
I return to my mantras about the antivaccine movement in the age of COVID-19: There is nothing new under the sun , and everything old is new again . Even the most obscure antivaccine pseudoscience and conspiracy theories are being resurrected, dusted off, tweaked a bit, and repurposed to use to attack COVID-19 vaccines. There doesnt need to be any evidence or even biological plausibility, just science-y speculation that sounds impressive to people without a background in the relevant sciences. Classens claim that mRNA vaccines can cause prion disease leading to neurodegenerative disorders like Alzheimers disease is just another example of this.
With Classens claim, Im hard pressed to think of an old antivaccine trope that hasnt yet been weaponized against COVID-19 vaccines, with one exception. No one, to my knowledge, has yet claimed that COVID-19 vaccines cause autism. The reason for that omission is simple, however. As of today, no COVID-19 vaccine has been authorized for use in children. As soon as a COVID-19 vaccine is authorized or approved for use in children, antivaxxers will claim it causes autism. Its coming. You know it is.
Recommended Reading: Diseases Similar To Parkinsons
Molecular Link Between Parkinsons And Prion Diseases Discovered
New research has proven the existence of an important interaction between the molecules involved in the two types of pathologies.
Parkinsons disease and prion diseases are very different from each other in regards to both origins and course. A group of researchers has discovered an unexpected and important link between the two pathologies.
According to the study recently published Scientific Reports, the link is a result the complex interaction between two different proteins present in our nerve cells: -synuclein, in its aggregated form, and the prion protein PrPC, the molecule which is responsible for Creuzfeldt Jacob disease.
The presence of -synuclein deposits in brain cells is typical of diseases technically called synucleinopathies, including Parkinsons disease, dementia with Lewy bodies and multiple system atrophy. However, the modalities according to which these aggregates form and spread were unknown. The study reports -synuclein actually makes use of the action of the prion protein to spread and deposit in the brain. This seems to favour the formation of these deposits and their spreading among brain cells.
What Is The Prion
Why did it take so long to embrace the idea that the propagation of protein aggregates is important in PD pathogenesis? Textbooks still classify PD as a movement disorder, and the most conspicuous signs and symptoms of PD involve akinesia, rigidity, and tremor. Most of these motor symptoms are due to reduced striatal dopamine, as a consequence of progressive degeneration of substantia nigra dopaminergic neurons, and dopaminergic drug therapies are initially effective at alleviating these symptoms. Because of the central role of the dopamine deficit in the most conspicuous PD symptoms, most research into underlying pathogenic mechanisms over the past 50 years has focused on the dopamine neurons and their putative selective vulnerability. However, patients also experience a wide range of troublesome nonmotor symptoms , and many of these are not linked to the reduction in striatal dopamine and therefore do not respond well to dopaminergic drug therapies .
Recommended Reading: Judy Woodruff Health Problems
Vaccines And Alzheimers Disease: A Brief History
One of the earliest antivaccine claims that I ever dealt with was a rather specific claim about the influenza vaccine. I first encountered it when Bill Maher parroted it in an interview with Larry King on Larry King Live, way back in December 2005. I think its useful to recount what Maher said in this exchange:
MAHER: Im not into western medicine. That to me is a complete scare tactic. It just shows you, you can
KING: You mean you dont get a you dont get a flu shot?
MAHER: A flu shot is the worst thing you can do.
KING: Why?
MAHER: Because its got its got mercury.
KING: It prevents flu.
MAHER: It doesnt prevent. First of all, thats
KING: I havent had the flu in 25 years since Ive been taking a flu shot.
MAHER: Well, I hate to tell you, Larry, but if you have a flu shot for more than five years in a row, theres ten times the likelihood that youll get Alzheimers disease. I would stop getting your
KING: What did you say?
MAHER: That went better in rehearsal but it was still good. Absolutely, no the defense against disease is to have a strong immune system. A flu shot just compromises your immune system.
A chiropractor. Of course, the presentation at NVIC had to be recorded by a chiropractor:
This is an old claim, dating back 24 years!
And heres a more recent addition since 2005, dated 2006:
But what about prion disease? And what are prion diseases, anyway?
What Are Prion Disorders
I have no difficulty with the concept of prion disorders. I think of prion disorders as protein cancers. Prions are proteins which are normally present in the body and perform essential functions. However, if they come into contact with a particular toxin or heavy metal or another twisted prion , then they too twist and distort. When they twist in such a way that they cannot be broken down by the body enzyme systems, they cause problems because the body cannot break them down so it dumps them. Pathologically this is known as amyloid. This results in deposition of these indigestible proteins and this can be anywhere in the body.
Cancers, of course, are simply cells which replicate themselves and build up to cause problems. Viruses are strips of DNA which replicate themselves and build up in the body to cause problems. Difficulties arise when they literally get in the way of the body and stop it functioning normally. Although amyloid can occur anywhere in the body, the biggest problem is in the brain, perhaps because it is a closed box and therefore there is not any room for all this excess protein to be dumped and partly because each part of the brain is unique and any loss of function is quickly noticed.
So these protein cancers tend to cause problems which may take many years to develop and so far the medical profession has no method of slowing down this process.
Don’t Miss: Fitflop Shoes For Parkinson’s
Evidence For Different Strains Of
Monomeric -SYN is considered natively unfolded as it populates a very large ensemble of conformational states that are affected by physical-chemical conditions . Each assembly-competent conformational state of -SYN monomers exposes specific amino acid stretches that determine its ability to establish defined sets of intermolecular interactions. These interactions lead to assemblies that exhibit different intrinsic structures and have distinct amino acid stretches exposed at their surfaces. The intermolecular interactions, lateral and longitudinal, that maintain -SYN molecules within the assemblies govern the ability of a given -SYN assembly to grow by incorporating additional monomeric -SYN molecules in well-defined conformations and in a thermodynamically stable manner. Importantly, the exposed amino acid stretches influence with which partner proteins, receptors, and lipids a given -SYN assembly will interact. Thus, it is evident that this can result in highly specific biological properties of a given -SYN assembly. In essence, the exposed amino acid in a specific type of -SYN assembly dictates its seeding propensity, resistance to cellular clearance machineries, cytotoxicity, tropism for different cell types in the nervous system, etc. Therefore, the concept of different -SYN fibril strains is very important for our understanding of the molecular pathogenesis of PD and other synucleinopathies.