Involvement Of Parkin In Mitochondrial Processes
As an E3 ligase, Parkin mediates both multiple monoubiquitination and polyubiquitination of its substrates . Parkin can assemble canonical and noncanonical ubiquitin chains . Upon binding to the protein substrates, the K48-linked ubiquitin targets them for proteasomal degradation, while other linkages can modify protein interactions, activity and localization .
Parkin interacts with and promotes ubiquitination of various proteins, mediating a diversity of cellular processes under basal and stress conditions, although no specific target sequence has been found for its recognition of substrates, suggesting that the specificity of Parkin is driven by its proximity to a substrate . This concept is supported by observations that exogenous proteins targeted to mitochondria are ubiquitinated by activated Parkin and when Parkin is recruited to peroxisomes by ectopic PINK1, it can efficiently drive ubiquitination of their surfaces . Particularly, Parkin activity is tightly connected to diverse aspects of mitochondrial functioning, and some of these processes will be reviewed in the following subsections .
Fig. 3
Relevance Of Mitochondria For Cellular Homeostasis
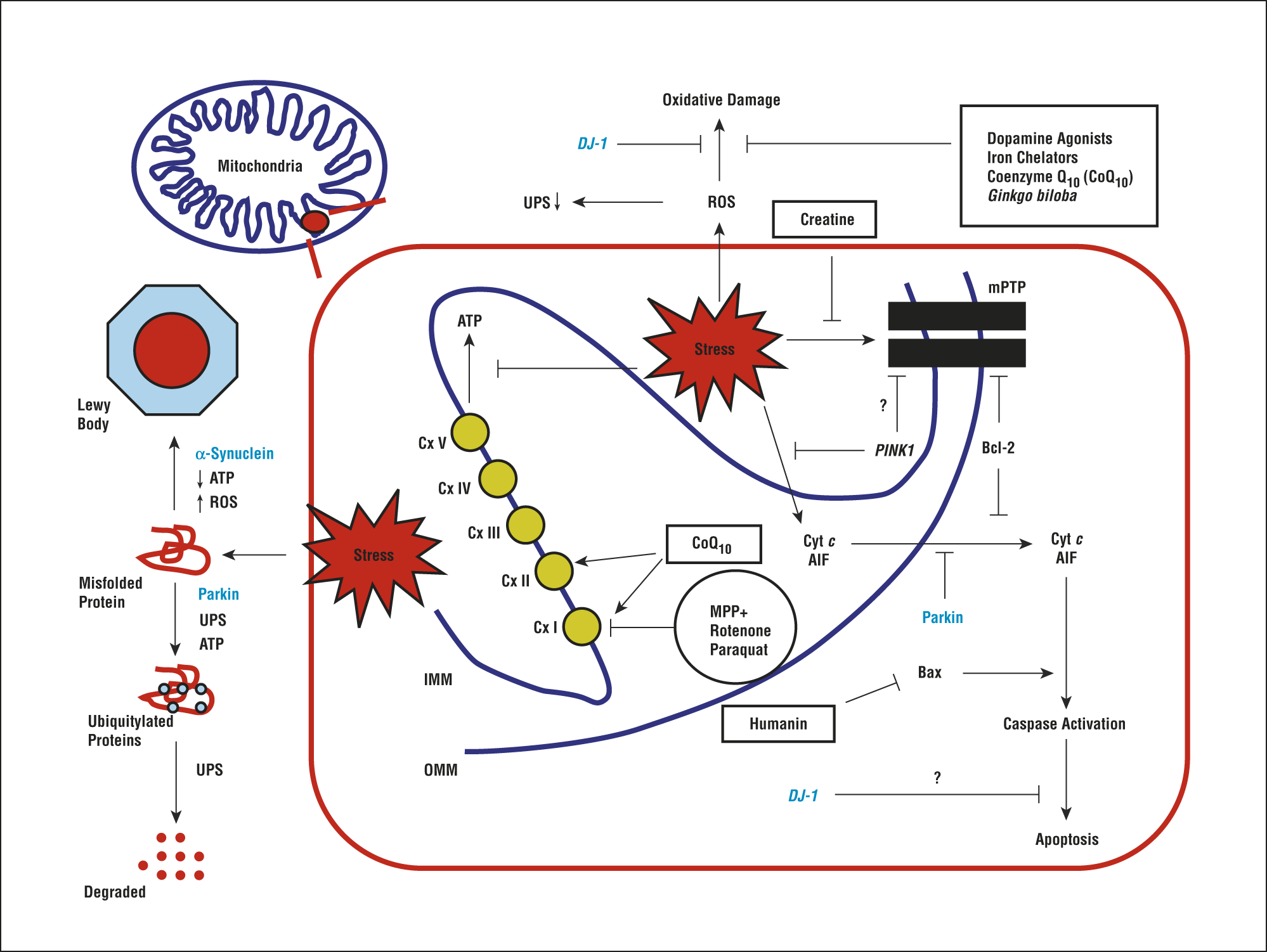
Mitochondria are highly dynamic organelles that are essential to maintain cellular function. These organelles maintain neuronal function and integrity via sustained energy supply for important cellular functions including synaptic activity or calcium buffering after depolarisation . Consequently, a tight regulation of mitochondrial homeostasis especially in neurons is necessary to maintain cellular processes. For example, reactive oxygen species play a role in mitochondrial signalling but if the concentration gets out of range, oxidative stress might arise and damage biological molecules and structures like DNA, proteins or lipid membranes . The production of adenosine triphosphate by oxidative phosphorylation in the mitochondrial electron transport chain is one of the key mitochondrial functions to provide energy. This process is accompanied by the passage of protons in the inter-membrane space, which subsequently creates the mitochondrial membrane potential . In case of an imbalanced MMP, production of a high level of ROS such as superoxide may arise due to electron leakage. Thus, the mitochondrion has to ensure a balance to efficiently produce ATP without releasing a pathological level of ROS.
Fig. 2
The Many Faces Of Etc Proteins: Functions Beyond Bioenergetics And Possible Implications In Neurodegeneration
Abundant studies have focused on mitochondrial quality control, lysosomal functions, apoptosis, and -Syn aggregation in PD pathogenesis and their influences on ETC functions. In contrast, whether ETC proteins have any direct involvement in these pathways other than bioenergetics remains unclear in neurodegenerative diseases.
The roles of ETC proteins in apoptosis have been extensively studied in cancer cell biology. Both cI and cII are implicated as apoptotic sensors via different mechanisms. In cI, cleavage of NDUFS1 by caspase-3 inhibits cI activities, causing ROS production and collapse of mitochondria membrane potential. The cleavage-resistant mutant NDUFS1 D255A decreases ROS formation and delays the loss of plasma membrane integrity , suggesting cI inhibition as an accelerator of apoptosis. A similar mechanism is found in granzyme A-induced apoptosis, in which NDUFS3 was cleaved . For cII, the acidification of matrix in response to mitochondrial stress causes the disintegration of cII by dissociating the anchoring subunit SDHC-SDHD from the enzyme subunit SDHA-SDHB. As the SDHA-SDHB subunit is still enzymatically active, it produces excessive ROS and causes apoptosis. cII, therefore, is a pH sensor of the matrix in programmed cell death.
Also Check: Cleveland Clinic Parkinson’s Bicycle Study 2017
Mitochondrial Dysfunction Affecting Cellular Integrity
In cases of sustained mitochondrial dysfunction, apoptosis of the cell is the last solution to avoid general damage to the organism. Apoptosis is a physiological event occurring during development and arising when molecular and organellar quality controls are overwhelmed. Two distinct pathways have been described, the extrinsic and the intrinsic pathways, with the latter involving a mitochondrial signalling pathway. Here, upon cellular stress, Bax and Bak are translocated in the mitochondrial OM where these proteins colocalise with mitochondrial fission sites . In order to trigger apoptosis, these pro-apoptotic proteins need to outbalance anti-apoptotic proteins like Bcl-2 and Bcl-xL . Bax and Bak then contribute to the release of cytochrome c through the permeability transition pore . Cytochrome c will subsequently form a complex with pro-caspase-9, which activates caspase-9, the initiator caspase. Caspase-9 will in turn promote the activation of caspase-3, the executioner caspase and lead to the activation of the apoptosis pathway.
VPS35 also has an anti-apoptotic role via its association with Lamp2a and with the Parkin substrate, aminoacyl-tRNA synthetase complex interacting multifunctional protein-2 . For VPS35 harbouring the PD-associated D620N mutation, this association was disturbed and led to an increased level of non-degraded AIMP2, which translocates to nucleus and activates PARP1 leading to cell death .
Origins Of The Link Between Mitochondria And Pd
First, the so-called frozen addicts suggested a contribution of mitochondrial dysfunction to the pathogenesis of PD. In these drug users, living in California in the 1980s, physicians observed that a side product of new synthetic heroin triggered a rapid onset of a distinct form of parkinsonism responsive to levodopa treatment. It turned out that the synthesis process resulted in the unwanted generation of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine , which led to inhibition of the respiratory chain . Of note, a similar observation was published already four years earlier . MPTP is not toxic itself but lipophilic and thus able to enter brain tissue by crossing the blood brain barrier. In the brain, it is processed by monoamine oxidase B to the toxic cation 1-methyl-4-phenylpyridinium . MPP+is selectively taken up by dopaminergic cells and inhibits multiple complexes of the respiratory chain . The notion that mitochondrial dysfunction plays a role in PD pathogenesis was supported shortly after the description of the frozen addicts by the observation of a restricted function of respiratory chain complexes in postmortem brain sections from PD patients . These early findings significantly stimulated PD research in the following years. For example, even today, the injection of MPTP is most commonly used to model PD in mice . However, similar to other animal models of PD, the clinical and pathological characteristics simulated by the MPTP model differ from PD in many ways .
Don’t Miss: Parkinson’s Double Vision
Mitochondria And Protein Aggregation
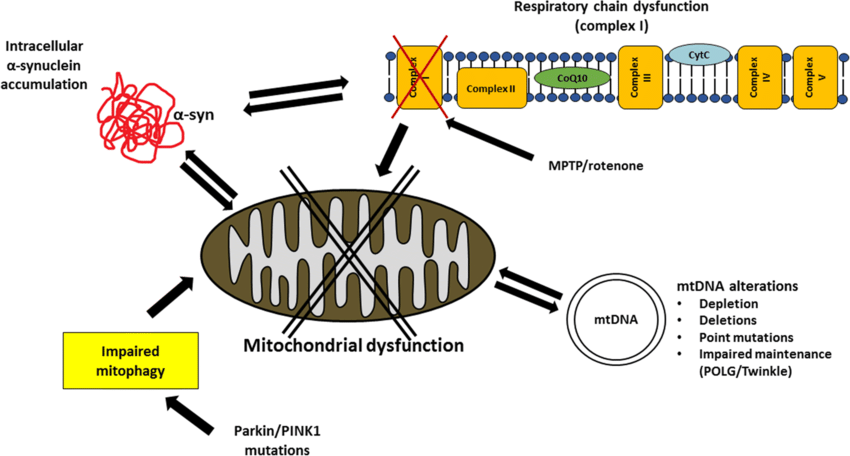
Alpha-synuclein has been shown to cause fragmentation of the mitochondrial network in a number of models . This fragmentation is DRP1 independent, occurs in the presence of both wild-type and mutant forms of the protein, and has been shown to precede the loss of mitochondrial function and neuron death. Interestingly, it has been suggested that the protein has no effect on the structure of the endoplasmic reticulum, the Golgi, or their interactions with the mitochondria. However, a recent study has shown that alpha-synuclein does interact with VAPB, an integral ER protein. This interaction then weakens the association of mitochondria with the ER, causing alteration in calcium buffering and the production of ATP . The effect of alpha-synuclein on the structure of the mitochondria extends beyond merely affecting their fission and fusion with alterations to the ultrastructure of mitochondria also reported. Kamp et al. showed that PINK1, Parkin, and DJ-1 over expression was sufficient to be able to rescue the effect of alpha-synuclein on mitochondrial dynamics .
Ros Production And Oxidative Stress
In addition to mitochondrial ROS, other factors that may contribute to increased oxidative damage include iron accumulation and an increase in lipid peroxidation burden , while accumulation of hydroxyl and superoxide radicals due to a decline in glutathione content have also been reported in the brain of PD patients. Recent studies also highlighted a direct toxicity of alpha-synuclein oligomers on mitochondria via induction of mitochondrial lipid peroxidation and oxidation of ATP synthase . It was shown that alpha-synuclein leads to increased permeability of mitochondrial membranes and ROS production, ultimately leading to neuronal death .
Don’t Miss: What Foods Should Be Avoided When Taking Levodopa
Does Mitochondrial Turnover Drive The Development Of Neurodegeneration In Pd
Although the process is still not clearly defined in neurons, data suggests that the degradation of dysfunctional mitochondria occurs through the autophagy related pathway, mitophagy. A number of studies have suggested that there is a decline in mitophagy with age . Furthermore, many of the genes that control the degradation of mitochondria through this pathway have been linked to ageing and lifespan in model organisms . A decline in lysosome function has also been shown to occur with advancing age, mainly through the accumulation of lipofuscin within these organelles . However, the fact that many of the genes responsible for familial PD have been shown to have important roles in autophagy or lysosomal pathways suggests that this is an important driver of the pathogenesis of PD. Alterations in the efficiency of such pathways will clearly exacerbate mitochondrial dysfunction, allowing defective mitochondria to accumulate. Mitochondrial dysfunction itself may also increase oxidative damage to proteins and organelles which may overwhelm these systems in PD, leading to neuronal loss. Again, we are faced with a vicious circle of damage, whereby mitochondrial dysfunction may be caused by defects in genes such as ATP13A2. However, lysosomal dysfunction may increase oxidative stress, which would then cause further mitochondrial and cellular damage.
Do Mitochondrial Dynamics Drive The Development Of Neurodegeneration In Pd
Together these data suggest that many of the proteins that are known to be integral to the development of PD have now been shown to have crucial roles in maintaining the dynamics, distribution, and transport of mitochondria within neurons. We are now beginning to understand more about these dynamic aspects of mitochondria due to improved imaging techniques and models. It is clear, however, that the correct distribution of mitochondria within neurons is as important to their function as the interconnectivity of these organelles. Furthermore, it is not only the movements of functional mitochondria which need to be monitored and controlled, but also the localisation and transport of those which are dysfunctional and require degradation. Disruption of these processes in many models has been linked with impaired neuronal function or even neurodegeneration. With advancing age and in PD the neurons of the SN do accumulate mitochondria which are dysfunctional and may therefore be unable to provide the required level of ATP. Failure to remove these mitochondria from important sites such as the synapse will then lead to further detrimental changes and neuronal loss.
You May Like: Parkinson’s Bike Therapy
Does Mitochondrial Dysfunction Drive The Development Of Neurodegeneration In Pd
It is evident from other conditions, for example, mitochondrial disorders associated with mutations in the polymerase gamma gene, POLG, that a primary mitochondrial defect is sufficient to cause loss of SN neurons . Furthermore, such mutations are often associated with the development of PD-like symptoms . However, attempting to disentangle cause and effect in the contribution of mitochondrial dysfunction to the pathogenesis of PD is difficult, since many of the changes we detect in those with PD are also present in healthy aged individuals. The mitochondrial defects present in these individuals often exist in the absence of cell loss or parkinsonian symptoms. For example, equivalent levels of mtDNA deletions are detected in SN neurons from individuals with PD and healthy controls and neurons showing deficiencies for both Complex I and Complex IV are also detected in both instances . Many of the processes described above initiate changes in other pathways and thus activate well defined responses which exist to mitigate to these changes, for example, ROS and antioxidants. Therefore, it might be suggested that in PD it is the ability of neurons to respond to mitochondrial functional changes that is impaired. Thus, neurons become more sensitive to changes in other pathways, which ultimately leads to their degeneration and loss.
Researchers Show Protein Controls Process That Goes Awry In Parkinsons Disease
National Institutes of Health
Disruptions in the fission of mitochondria, the structures within cells that make energy, are behind a host of other ailments, including cancer, diabetes and heart disease.
As scientists work toward finding a cure for Parkinsons disease, one line of research that has emerged focuses on mitochondria, the structures within cells that make energy. The health of those structures is maintained through a quality control system that balances two opposite processes: fission one mitochondrion splitting in two and fusion two becoming one.
When theres a problem with fission, that system is thrown out of balance. The consequences can include neurodegenerative diseases, such as Parkinsons disease, and other serious conditions.
For years, scientists have known that one particular protein, called Drp1, is a master regulator of mitochondrial fission, but little else about how Drp1 is controlled by other proteins. Essential processes in biology are governed by complex biochemical chain reactions among such proteins. Scientists call these chain reactions signaling pathways.
Now, a research collaboration led by UCLA investigators has brought new light to the mechanisms controlling Drp1 and the fission of mitochondria. The findings could advance the fight against Parkinsons and a variety of other diseases.
Guos research group is continuing to investigate how CLUH controls mitochondrial fission and its impact in cellular and organism health.
Also Check: Prayers For Parkinson’s Disease
Linking Mitochondrial Dysfunction With Pd
The mitochondrial theory of PD is based on observations in which disease processes impairing oxidative phosphorylation, mitochondrial biogenesis or mitophagy manifest as phenotypes that share common Parkinsonian features. The first link between PD and mitochondrial dysfunction was conceived by Langston after observing rapidly developing Parkinsonian features in intravenous drug abusers who were exposed to 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine , a toxin whose metabolite 1-methyl-4-phenylpyridinium inhibits mitochondrial complex I . Once in the central nervous system, MPP+ is taken up by the dopamine active transporter and induces the selective degeneration of SNpc dopaminergic neurons. This phenomenon has been successfully reproduced in MPTP-treated rodents and nonhuman primates, popularizing these animal models for PD research . Complementing the findings in MPTP, rotenone, a pesticide that inhibits mitochondrial complex I, produced similar PD-like locomotor deficits in treated animals. Despite it having widespread uptake in the central nervous system, the toxic effects of rotenone are most pronounced in SNpc dopaminergic neurons, reinforcing the belief that neurons with greater susceptibility to energy perturbances would be affected the most . Further evidence is gleaned from epidemiological studies, which identified rotenone-exposed agrarian populations to be at greater risk of developing PD .
Mitochondrial Fusion And Fission
The hundreds of mitochondria within a cell undergo continual cycles of fusion and fission , resulting in a wide range of mitochondrial morphologies . The adequate balance between fusion and fission is crucial for the maintenance of mitochondrial function. For instance, mitochondrial fusion is required for the proper respiratory activity of the mitochondria and has been associated with cell survival . In addition, the functionality of damaged mitochondria can be restored by exchanging mitochondrial genomes and gene products by fusion with neighboring, intact mitochondria, thereby attenuating the potential deleterious effects of misfolded proteins or mutated mtDNAs . The proper localization of mitochondria to nerve terminals also depends on the correct balance between mitochondrial fusion and fission, as fragmentation of the mitochondrial network by fission appears to facilitate the recruitment of mitochondria to nerve terminals .
You May Like: Does Sam Waterston Have Parkinsons
Mitochondrial Quality Control Mechanisms
Studying PINK1- and Parkin-associated PD, the most frequent autosomal recessive forms of the disease, has been discovered that these two genes are the main responsible of mitochondrial quality control maintenance, working at various levels. Indeed, they participate both at a molecular level for mild mitochondrial dysfunction and, in a stepwise manner, an organellar level for more severe mitochondrial dysfunction. The former involves molecular chaperones and proteases to correct protein import and folding and to regulate turnover the latter aims to sequester, sort, and eliminate partially or completely damaged mitochondria. Organellar level involves fusion, fission, and mitophagy processes as well as mitochondrial-derived vesicles pathway.
The failure of both molecular and organellar levels of mitochondria QC mechanisms leads to irreversible damage of mitochondria and consequent release of its components and proapoptotic proteins into the cytosol. These events, by the apoptotic pathway, lead to cell death , which could also be strengthened by the activation of neuroinflammatory mechanisms . Indeed, the release into the cytosol of mtDNA together with other compartmentalized mitochondrial molecules could activate a Toll-like receptor 9-mediated inflammatory response , which induces the production of interleukin -1 and other proinflammatory cytokines . These events could represent the way by which PINK1 and Parkin mutations provoke loss of dopaminergic neurons and PD.
How Does Mitochondria Contribute To Parkinsons
Research in the last few decades has confirmed that there is a direct link between mitochondria and Parkinsons disease. And its now generally accepted that mitochondria play a big role in the development of Parkinsons disease.
There are two main ways through which mitochondria contribute to Parkinsons disease. One is the mutations in genes linked to mitochondria. The other is through the generation of reactive oxygen species that cause damage to vital components of brain cells, especially those involved in dopamine production.
Recommended Reading: Diseases Similar To Parkinsons
Enhancing The Clearance Of Dysfunctional Mitochondria Via Mitophagy Or Other Mitochondrial Stress Response Pathways
Multiple lines of evidence point to the importance of mitophagy in the pathophysiology of PD. For example, the PRKN and PINK1 genes mediate mitophagy and are the major causes of autosomal recessive early onset mPD . Therefore, enhancing mitophagy is a key therapeutic strategy in PD . In keeping with this, investigators used a rodent model of PD to study the effect of kinetin, the precursor of kinetin triphosphate, an activator of both wild-type and mutant forms of PINK1 . However, in PINK1 null rodents, no degeneration of midbrain dopamine neurons was identified. Additionally, in rodent models of -synuclein induced toxicity, boosting PINK1 activity with oral kinetin provided no protective effects, thus showing no evidence of a beneficial effect in a preclinical model of IPD . Another agent, celastrol, was shown to exert neuroprotective effects through activating mitophagy and inhibiting dopaminergic neuronal loss in PD cell and mouse models . Recently, a study used a high-throughput phenotype detection system for drug screening in dopaminergic neurons from induced-pluripotent stem cells derived from patients with PD due to PRKN or PINK1 mutations . After screening 320 compounds, they identified 4 candidate drugs that were effective for ameliorating impaired mitochondrial clearance, showing the utility of this method for identifying candidate PD drugs .