Selection Criteria And Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Selection Criteria.
Am I A Good Candidate For Dbs
To determine if you are a good candidate, you:
Page reviewed by Dr. Chauncey Spears, Clinical Assistant Professor and Dr. Amelia Heston, Movement Disorders Fellow at the University of Michigan.
Research To Improve Deep Brain Stimulation
Researchers are working to improve upon existing DBS devices and methods to help treat more symptoms and more people. Some researchers are putting electrodes in a different area of the brain the pedunculopontine nucleus to treat walking and balance problems that don’t typically improve with present-day DBS. Others are developing a “smart” DBS device that can record a person’s unique brain signals and deliver electrical stimulation only when needed, such as when symptoms return, rather than continuously, as the current systems do. This could help reduce side effects such as numbness and weakness and lengthen the battery life of the neurostimulator, which would result in a longer time between battery replacement procedures.
Scientists also are planning to test deep brain stimulation in the first years after a Parkinson’s diagnosis to see if the therapy may slow or stop disease progression. Testing in Parkinson’s models showed the therapy may help protect brain cells, and a small human trial showed motor symptoms improved after early-stage DBS.
Don’t Miss: How Does A Doctor Diagnose Parkinson’s Disease
Guidelines With Unclear Methodology
- AndersonDG, Van CollerR, CarrJ. South African guideline on deep brain stimulation for Parkinsons disease. S Afr Med J. 2017 107:10271032.
- FoxSH, KatzenschlagerR, LimSY, et al. International Parkinson and movement disorder society evidence-based medicine review: update on treatments for the motor symptoms of Parkinsons disease. Mov Disord. 2018 33:12481266.
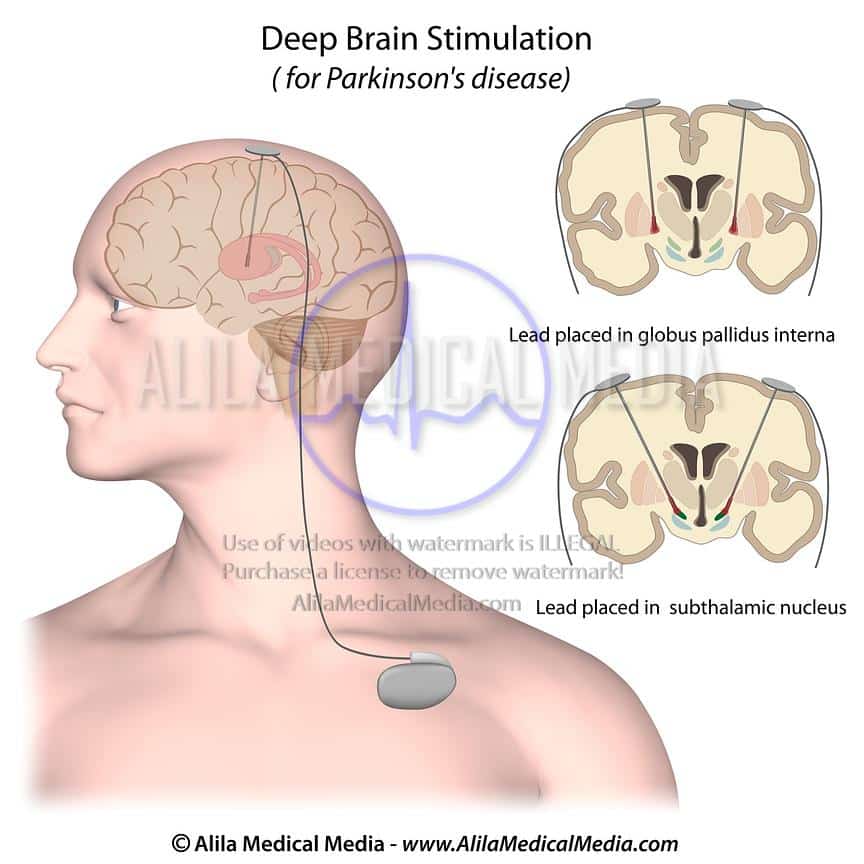
How Does Deep Brain Stimulation For Parkinsons Work
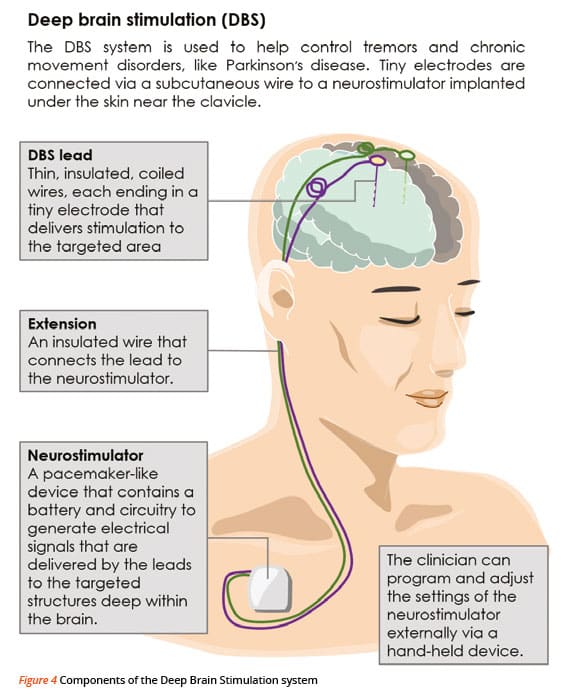
Deep brain stimulation works by modifying abnormal electrical activity in the brain. It was first approved for Parkinsons tremors in 1997 and has become an established treatment to control additional motor symptoms of Parkinsons disease.
DBS involves three main components:
- Leads: Leads are implanted in the brain in a region responsible for motor activity.
- Implantable pulse generator : A separate procedure is performed to implant a battery-operated device in the chest or in the abdomen. An IPG is similar to a pacemaker for the heart and has been coined by some as a pacemaker for the brain.
- Extension: A thin, insulated wire is passed beneath the skin between the leads and implantable pulse generator to deliver the electrical stimulation from the pulse generator to the leads.
The target area in the brain is first identified by magnetic resonance imaging or computed tomography . Then, the leads are placed via small holes that a surgeon drills in the skull.
This is considered a minimally invasive surgery that is done in the operating room with local anesthesia. It usually requires an overnight stay.
The IPG is inserted in a separate surgical procedure in the operating room roughly a week later.
After a few weeks, a neurologist begins to program the unit. This process can take several additional weeks to months. When this is completed, people are able to manage the device with a handheld remote control.
Don’t Miss: Parkinson’s Disease And Vision Problems
Stereotactic Dbs Vs Interventional Image
Stereotactic DBS surgery requires the patient to be off their medication. During the procedure, a frame stabilizes the head and provides coordinates to help the surgeons guide the lead to the correct location in the brain. The patient gets local anesthesia to keep them comfortable throughout each step along with a mild sedative to help them relax.
During image-guided DBS surgery, such as with interventional MRI or CT scan, the patient is often asleep under general anesthesia while the surgeon uses images of the brain to guide the lead to its target.
Some advanced centers offer both the stereotactic and iMRI-guided options for DBS surgery. In this case, the doctor and patient will discuss which procedure is better based on a number of factors.
For instance, the doctor may recommend an image-guided procedure for children, patients who have extreme symptoms, those who are especially anxious or fearful or those whose leads are going into certain parts of the brain.
Generally, DBS surgery follows this process:
Inclusion And Exclusion Criteria
For our analysis, we included observational clinical studies that compared GPi-DBS vs. STN-DBS, or GPi vs. medical therapy, or STN-DBS vs. medical therapy in patients with advanced PD.
The following inclusion criteria were used: clinical trials of DBS for treatment of idiopathic PD study subjects: patients clinically confirmed as PD study outcomes: studies that used the UPDRS score to assess the post-treatment results outcomes in those studies were measured more than 3 months post-surgery and contained clear reports of medication phases.
The exclusion criteria were: DBS was performed in patients with pathologies other than PD they were not concurrent, controlled clinical studies data could not be extracted or lacked data integrity involved complex intervention strategies.
Also Check: How Long Do Elderly Live With Parkinson’s
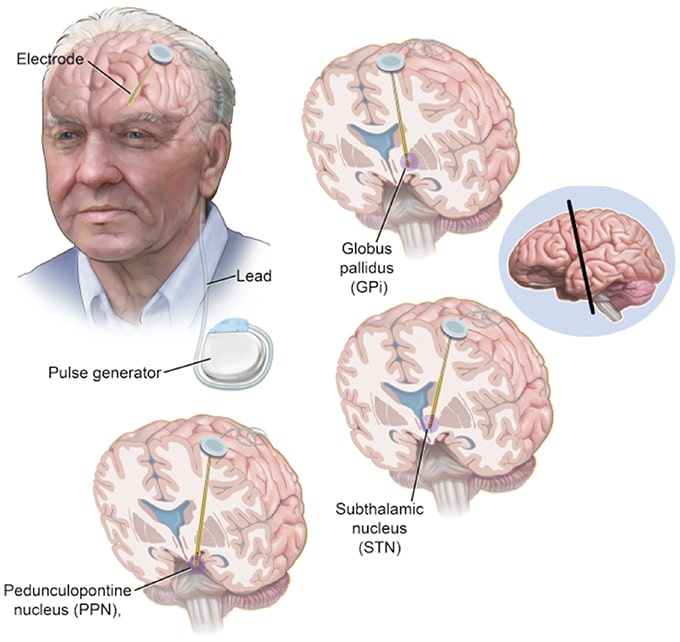
Sites Of Deep Brain Stimulation And Symptom Control
While both subthalamic nucleus and globus pallidus internus stimulation help improve the motor symptoms of Parkinsons disease, studies have found a few differences.
DBS of the third target, the ventral intermediate nucleus, can be beneficial for controlling tremors but does not work as well at addressing the other motor symptoms of Parkinsons disease.
In a Canadian study, targeting the subthalamic nucleus allowed people to reduce the doses of their medications to a greater degree, while targeting the globus pallidus internus was more effective for abnormal movements .
In another study, STN deep brain stimulation also led to a greater reduction in medication dosages. However, GPi stimulation resulted in greater improvement in quality of life, and also appeared to help with the fluency of speech and depression symptoms.
Side effects of DBS can sometimes include subtle cognitive changes . A different study compared these effects with regard to these different areas.
GPi showed smaller neurocognitive declines than STN, though the effects were small with both. On a positive note, both procedures seemed to reduce symptoms of depression following surgery.
Critical Appraisal Of Individual Studies
The included systematic reviews were critically appraised by one reviewer using A MeaSurement Tool to Assess systematic Reviews 2, randomized studies were critically appraised using Downs and Black checklist, economic studies were assessed using the Drummond Checklist, and guidelines were assessed with the Appraisal of Guidelines, Research and Evaluation II instrument. Summary scores were not calculated for the included studies rather, a review of the strengths and limitations of each included study were described narratively.
You May Like: Insomnia And Parkinson’s Disease
Deep Brain Stimulation Care For Movement Disorders
At the USC Deep Brain Stimulation Center, we provide care to decrease the symptoms of dystonia, essential tremor and other movement disorders, such as Parkinsons disease. We use deep brain stimulation when other medical and psychological treatments do not improve your condition.
Deep brain stimulation acts like a pacemaker for your brain. The device blocks abnormal electrical signals in your brain that can cause uncontrolled movements. It can also improve mobility for people who are slowed down by Parkinsons disease. It doesnt cure movement disorders, but it does relieve symptoms so you can be more independent and comfortable.
Key Publications Of Alim Louis Benabid
Benabid, A.L., Pollak, P., Louveau, A., Henry, S., and de Rougemont, J. . Combined stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl. Neurophysiol. 50, 344-346.
Benabid, A.L., Pollak, P., Gervason, C., Hoffmann, D., Gao, D., Hommel, M., Perret, J.E., and de Rougemont, J. . Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 337, 403-406.
Limousin, P., Pollak, P., Benazzouz, A., Hoffmann, D., Le Bas, J.F., Broussole, E., Perret, J.E., and Benabid, A.L. . Effect on parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 345, 91-95.
Limousin, P., Krack, P., Pollak, P., Benazzouz, A., Ardouin, C., Hoffmann, D., and Benabid, A.L. . Electrical stimulation of the subthalamic nucleus in advanced Parkinsons disease. N. Engl. J. Med. 339, 1105-1111.
Krack, P., Batir, A., Van Blercom, N., Chabardes, S., Fraix, V., Ardouin, C., Koudsie, A., Dowsey-Limousin, P., Benazzouz, A., Le Bas, J.F., Benabid, A.L., and Pollak, P. . Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinsons disease. N. Engl. J. Med. 349, 1925-1934.
Also Check: Parkinson’s Disease Physical Therapy Exercises
What Happens During Deep Brain Stimulation
This procedure actually involves two to three surgeries that usually happen at different times. The first one or two procedures are to insert the stimulation leads into each side of your brain at the same or separate times. The second procedure is to implant the stimulator battery known as a pulse generator under the skin of your upper chest.
Before these surgeries happen, your healthcare provider will usually insert an intravenous line to give you IV fluids. An IV also allows them to give you medications during the procedure as needed.
Lead placement
This procedure usually starts with your healthcare provider shaving the hair on your scalp. This makes it easier to place your head into a special frame that will hold your head still. The frame is set with four pins in your skull. This is done while youre under sedation, and you likely wont remember this part.
Once the frame is set, theyll bring in an intra-operative CT scanner to take images of your brain and identify the trajectory used for the electrode placement. Once the CT scan is complete, the entry point is identified, sedation is turned back on and your head is cleaned with surgical prep. Local anesthetic is then injected to numb that area of your scalp and skull. Your neurosurgeon will then make a small cut .
Pulse generator placement
Acceptance Remarks 2014 Lasker Awards Ceremony
I dont recall when I realized I wanted to do research, but I have always enjoyed understanding how things work. A growing fascination with how the brain controls behavior led me to medicine and then to neurology. This took a clear direction when I found a choice research position at the NIH in the laboratory of renowned researcher Edward Evarts. Because the other obvious brain regions were already assigned to other fellows, I was asked to work on the basal ganglia, a cluster of poorly understood brain structures, and to determine their role in the control of bodily movements.
Having spent much of my early career in both basic research and clinical work, I have been fortunate to see how the basic science contributes to patient care. I am also aware of how far this field, that we now call neuromodulation, has progressed.
Lasker Awards honor individuals who contribute critically to a research problem but they also highlight the larger progress in a given field, in our case, using targeted electrical stimulation to restore function in the disrupted neural networks responsible for both neurologic and psychiatric disorders.
I thank the Lasker Foundation I thank you all for giving me this award, and I hope to live up to it!
You May Like: Parkinson’s Disease Specialist In Florida
Center For Parkinson’s Disease & Other Movement Disorders
The Center for Parkinson’s Disease and Other Movement Disorders at NewYork-Presbyterian Brooklyn Methodist Hospital offers the only comprehensive diagnostic and treatment program for Parkinson’s and other movement disorders in Brooklyn.
Parkinson’s disease is a disorder of the central nervous system, involving the degeneration and loss of nerve cells in the basal ganglia of the brain. The disease occurs in both men and women and, while symptoms may occur as early as age 40, they are usually not apparent until patients are in their 60s or 70s.
What You Need To Know
- Surgeons implant one or more small wires in the brain during a surgical procedure.
- The leads receive mild electrical stimulation from a small pulse generator implanted in the chest.
- Proper patient selection, precise placement of the electrodes and adjustment of the pulse generator are essential for successful DBS surgery.
- DBS does not fully resolve the symptoms of PD or other conditions, but it can decrease a patients need for medications and improve quality of life.
Also Check: Ejercicios Para Personas Con Parkinson
Deep Brain Stimulation For The Treatment Of Parkinsons Disease And Other Movement Disorders
Parkinson’s disease is a neurodegenerative disorder that leads to resting tremor, rigidity, slowness of movement, and postural instability. These symptoms are caused by degeneration of neurons in the substantia nigra pars compacta , one of a group of brain structures known as the basal ganglia and part of a circuit crucial for coordinating purposeful movement. This circuit relies on the chemical messenger dopamine, which is produced by SNc neurons. As PD progresses and these neurons are lost, reduced dopamine results in abnormal circuit activity and motor symptoms.
The molecular precursor to dopamine, L-DOPA , is used to treat PD. However, people in later stages of the disease experience off periods when this medication does not work well, and L-DOPA treatment can also trigger uncontrolled involuntary movement, a condition called dyskinesia. deep brain stimulation can offer symptomatic relief in later stages of PD and may reduce requirements for L-DOPA treatment and exposure to its side effects. DBS is also used to treat other movement disorders, including essential tremor, which causes involuntary shaking that worsens during movement, and dystonia, which causes involuntary muscle contractions and slow, repetitive movements or abnormal postures.
Less Medication More Relief
Medtronic DBS therapy may reduce the need for other Parkinsons medications1 and, consequently, medication-related side effects. DBS delivers therapy 24 hours a day and doesnt wear off while sleeping. Its already working when you wake up.
* Signal may not be present or measurable in all patients. Clinical benefits of brain sensing have not been established.
You May Like: Parkinson’s Disease Charity Donations
Coordinates Of Active Electrode Contacts
Retrospective analysis of the stereotactic position of the active electrode contacts was done in 25 patients for whom postoperative T1 weighted MRI of sufficient quality or stereotactic radiographic examinations were available. Such analyses could not be undertaken in other patients implanted with subthalamic nucleus electrodes during the same period because of motion artefacts in the postoperative MRI, missing postoperative T1 weighted MRI, or missing postoperative stereotactic x rays. For the 25 patients evaluated, the mean and median coordinates of all active contacts are summarised in table 3. The mean laterality of all active electrode contacts mm median 12.7 mm) correlated well with the laterality of the subthalamic nucleus, as determined 3 mm ventral to the intercommissural plane in T2 weighted MRI of 35 patients mm). However, in the dorso-ventral direction the mean and median z coordinate of all active contacts do not project within the subthalamic nucleus proper, but suggest an area between the dorsal margin of the subthalamic nucleus and the subthalamic region according to different stereotactic brain atlases. Moreover, 12 of 49 active contacts were located within 0.5 mm of the intercommissural plane or further dorsal they were thus most probably in the subthalamic area.
Active electrode contacts relative to the mid-commissural point
His Right Leg Became Less Dyskinetic
Another positive change that Dad experienced after DBS was less overall dyskinesia, and especially in his right leg: When I used to come off of levodopa, my right leg would shake uncontrollably for 10 to 15 minutes, and thats gone away, he said. DBS totally took dyskinesia out of my right leg.
Since dyskinesia was one of the main reasons Dad decided to undergo DBS, I was happy to learn that it tackled some of his worst symptoms.
While Dad was initially worried about the potential negative side effects of undergoing DBS, hes a believer in the procedure now. I think the DBS works, and it continues, he said. It improves a little at a time. Its been four years, and it still functions quite well.
As for what Ive witnessed, I think Dad is experiencing a slower progression than he was before DBS. And for that, Ill be eternally grateful to his surgeon.
Recommended Reading: Common Side Effects Of Parkinson Medications
What Is Deep Brain Stimulation Or Dbs
Deep brain stimulation, or DBS, is often described as a pacemaker for the brain. It works much like a pacemaker, sending electrical signals to the brain instead of the heart. DBS is primarily utilized for patients who have Parkinsons disease, dystonia, or essential tremor, and who cant adequately control their disease with medication. Before any patient is considered for the surgery, they are evaluated by the U-M interdisciplinary team. That team includes a neurosurgeon, neurologist, clinical neuropsychologist, speech pathologist, social worker, and other team members who ensure that you and your family understand the procedure and discuss your expectations and concerns.
Its important to understand that DBS does not offer a cure for your disease, but a way to manage it more effectively. It can offer many benefits, including the need to take less medication and therefore experience fewer medication side effects.
Postoperative Mri Based Evaluation Of Deep Brain Stimulating Electrodes
Correlations of preoperative and postoperative MRI can be used to delineate the deep brain stimulating electrodes with respect to the subthalamic nucleus, as depicted by T2 weighted spin echo imaging. This is best done with MRI of high resolution and devoid of artefacts, a situation achievable by the acquisition of the preoperative images under general anaesthesia. As demonstrated for a representative patient in fig 2, the subthalamic nucleus can be clearly delineated in preoperative axial spin echo images as well as in coronal and sagittal reconstructions derived therefrom. The planning tracks are indicated by red lines and blue lines . The implanted electrodes can be visualised relative to the subthalamic nucleus using different image fusion modesthat is, a detail of the postoperative image shows part of the electrode overlaying the preoperative image , or intermediate weighting of images reveals both the electrode and the subthalamic nucleus .
Figure 2
MRI may also be valuable for the analysis of electrodes implanted in patients who previously had ablative procedures within the basal ganglia. In one patient a thalamo-subthalamotomy had been placed within and towards the base of the Vop according to the Schaltenbrand and Wahren atlas, which had been correlated with the T1 weighted MRI . In this patient deep brain stimulation was done in the vicinity of, but in an area clearly distinct from, the site lesioned previously.
Figure 3
You May Like: How To Control Parkinson Disease