Design Setting And Patients
The Long-term Study 1, a multicenter, double-blind, parallel-group, placebo-controlled, 1:1 randomized efficacy trial. Participants were recruited from 45 investigative sites in the United States and Canada and included 1741 men and women with early and treated Parkinson disease. Participants were enrolled from March 2007 to May 2010 and followed up until September 2013.
Vitamin C And Vitamin E
Vitamin C and vitamin E are both antioxidants. One study that evaluated these vitamins found they helped delay the need for PD drugs. Taking vitamin E alone did not seem to have the same benefit. However, vitamin E supplements can increase the risk of bleeding, especially in those who take blood thinners. 3,5
Vitamin E has also been studied for its potential to reduce the risk of developing PD. However, dietary intake of vitamin E did not show any reduction in the risk of developing PD.3,5
Main Outcomes And Measures
The primary outcome measure was a difference in clinical decline from baseline to 5-year follow-up, compared between the 2 treatment groups using a global statistical test. Clinical status was defined by 5 outcome measures: Modified Rankin Scale, Symbol Digit Modalities Test, PDQ-39 Summary Index, Schwab and England Activities of Daily Living scale, and ambulatory capacity. All outcomes were coded such that higher scores indicated worse outcomes and were analyzed by a global statistical test. Higher summed ranks indicate worse outcomes.
Read Also: Can You Recover From Parkinson’s Disease
Changes In Levels Of Creatinine And C
The patients with amyotrophic lateral sclerosis showed statistically significantly decreasing levels of creatinine from 1year before until 2years after diagnosis . Controls of amyotrophic lateral sclerosis patients had, on the other hand, increasing levels of creatinine during large part of these 4years, although the annual change was not always statistically significant. Patients with amyotrophic lateral sclerosis had increasing levels of C-reactive protein from diagnosis until 2years after diagnosis . Controls of patients with amyotrophic lateral sclerosis demonstrated, however, no clear change of C-reactive protein during the 4years.
Creatine For Parkinson’s Disease
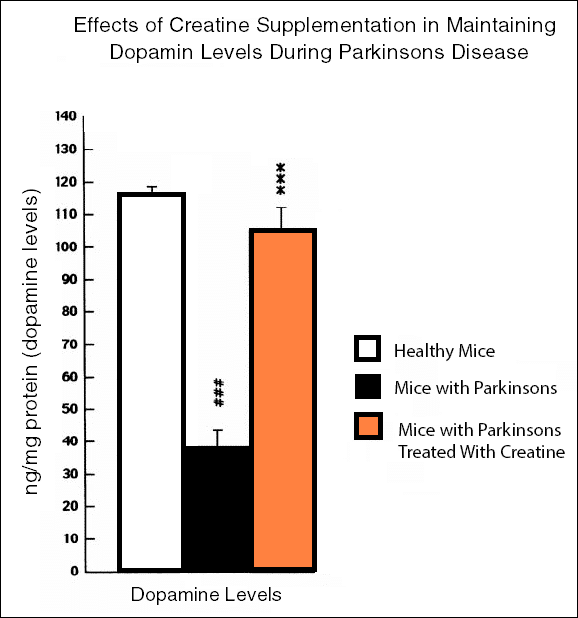
Parkinson’s disease is one of the most common neurodegenerative disorders and mitochondrial dysfunction plays an important role in its pathogenesis. Creatine has been shown to help improve mitochondrial function and may, therefore, useful for treating people with Parkinson’s disease. Researchers from the Cochrane Collaboration examined the evidence on whether creatine is effective and safe to treat people with Parkinson’s disease, used alone or as an adjuvant treatment, up to 10 November 2013.
We included two randomized controlled trials with a total of 194 patients that compared creatine with placebo for people with Parkinson’s disease. The effect of creatine on improvement of motor function, activities of daily living or quality of life after one or two years treatment for Parkinson’s disease was uncertain due to the low quality of the trials and the small number of participants they recruited. The serious adverse events that occurred in the trials were not thought to be related to creatine. However, one trial reported higher rates of gastro-intestinal effects with creatine.
The evidence base on the effects of creatine in Parkinson’s disease is limited by risk of bias, small sample sizes and short duration of the eligible trials. It does not provide a reliable basis on which treatment decisions can be made. Future well-designed RCTs with larger sample size and long-term follow-up are needed to assess creatine for Parkinson’s disease.
Don’t Miss: Parkinson’s Disease Eye Problems
Can Creatine Provide Protection Against Parkinsons Disease
Animal studies have shown that creatine has a neuroprotective ability and its consumption can protect the brain from developing Parkinsons disease. A study performed on mice showed that creatine supplementation protects the brain from MPTP-mediated dopamine depletion. MPTP is a neurotoxic chemical that when consumed cause the death of dopamine-producing cells in the brain and produces Parkinsons like symptoms in mice .
Such a neuroprotective effect of creatine has also been shown in another study. The study found that creatine in combination with Coenzyme Q10 not only restored dopamine level in the brain after MPTP treatment but also protect the brain from other Parkinsons disease pathogenic events like the accumulation of harmful proteins, lipid peroxidation and DNA oxidative damage .
Creatine Does Not Slow Rate Of Parkinsons Disease Progression
Treatment with creatine monohydrate for at least five years for patients with early and treated Parkinsons disease failed to slow clinical progression of the disease, compared with placebo, according to a study in JAMA.
Parkinsons disease is a progressive neurodegenerative disorder that affects approximately six million people worldwide. Incidence is expected to increase over the next decade, but neither a cure nor a treatment is available that has been proven to slow progression. Evidence indicates that creatine, an amino acid, plays an important role in cellular energy production, which may be impaired in Parkinsons disease. Oral creatine supplementation in mice has suggested a neuroprotective effect, according to information in the study.
Karl Kieburtz, of the University of Rochester, Rochester, USA, and colleagues, randomly assigned 1,741 men and women with early and treated Parkinsons disease to receive placebo or creatine monohydrate for a minimum of five years . Participants were recruited from 45 investigative sites in the USA and Canada, enrolled from March 2007 to May 2010, and followed up until September 2013.
There were no detectable differences in adverse and serious adverse events by body system.
These findings do not support the use of creatine monohydrate in patients with Parkinson disease, the authors conclude.
Recommended Reading: Symptoms Of Lewy Body Parkinson’s
Strengths Of The Trial
We enrolled 1741 participants and were able to retain more than 76% in this 5-year trial at the time the study was stopped. We chose novel measures of Parkinson disease progression because we believed no single outcome measure captured the progressive disability in Parkinson disease and also used a GST to combine information from these outcomes, giving us greater than 99% power to detect treatment effects. The chosen creatine dosage of 10 g/d was generally well tolerated. Despite early concerns that creatine exposure could be associated with deterioration of renal function or weight gain, long-term creatine use did not appear to adversely affect renal function or body mass index. The stabilization in creatinine levels that followed the initial rise, in the setting of continued creatine use, suggests that the initial increase represents an artifact of treatment rather than a sudden onset of renal disease.
Nih Announces Phase Iii Clinical Trial Of Creatine For Parkinsons Disease
Nutritional Supplement May Slow Progression of Disease.
The NIH National Institute of Neurological Disorders and Stroke today is launching a large-scale clinical trial to learn if the nutritional supplement creatine can slow the progression of Parkinson’s disease . While creatine is not an approved therapy for PD or any other condition, it is widely thought to improve exercise performance. The potential benefit of creatine for PD was identified by Parkinsons researchers through a new rapid method for screening potential compounds.
The double-blind, placebo-controlled, phase III study is one of the largest PD clinical trials to date. It will enroll 1720 people with early-stage PD at 52 medical centers in the United States and Canada.
“This study is an important step toward developing a therapy that could change the course of this devastating disease,” says Elias A. Zerhouni, M.D., director of the NIH. “The goal is to improve the quality of life for people with Parkinson’s for a longer period of time than is possible with existing therapies.” Currently there is no treatment that has been shown to slow the progression of PD.
“This study is an example of the Institute’s commitment to Parkinson’s research,” says NINDS director Story C. Landis, Ph.D. “We are trying to explore every possible option for reducing the burden of this disease.”
Avicena Group, Inc. will provide the creatine and the placebo for the study.
NIHTurning Discovery Into Health®
Read Also: Knee Replacement Surgery Recovery And Parkinson’s
Limitations Of The Trial
The observed annual rate of progression on the individual measures was slower than anticipated in our power analysis for Symbol Digit Modalities Test and PDSI , and as expected for the other measures . Variability in the rate of progression over 5 years was higher than anticipated,8 but the PDSI progression in another large Parkinson disease trial was similar.31 However, our failure to find a benefit was not attributable to reduced power, given the high power of the GST even in the presence of increased variability.
It is also possible that the study used too low a dose of creatine. Because the loss of the reliability of serum creatinine as a marker of kidney function is a likely adverse consequence of creatine use in clinical practice with older adults, we studied a total dosage of creatine used in the futility study. Another dosage could have different beneficial or harmful effects however, concerns about tolerability and masking of adverse kidney consequences limited the dosage used in the study.
With respect to adherence, at the time of analysis 34% of participants randomized to creatine had stopped medication and 5% had stopped per protocol. Only 26% of those randomized to placebo stopped medication, and less than 1% stopped per protocol. A completers analysis of the subset continuing to take their medication for at least 4 years and with a 5-year visit gave results similar to analysis of the ITT cohort.
Study Recruitment And Retention
Enrollment occurred from March 13, 2007, to May 28, 2010. Eligible participants were fewer than 5 years from Parkinson disease diagnosis and had taken levodopa or a dopamine agonist for at least 90 days but not longer than 2 years. Continuation of other prescribed Parkinson disease therapy was allowed. Participants were to be followed up for a minimum of 5 years or until the end of the trial and encouraged to remain in the study even if they discontinued study drug. Adjustments of Parkinson disease medication were permitted during the trial. The institutional review board approved the study, the study protocol, and the informed consent process and documentation. All patients provided written informed consent.
Also Check: Who Does Parkinson’s Disease Affect
Creatine Doesn’t Slow Parkinson’s Progression
Pauline Anderson
Despite early promise and a great deal of interest in creatine monohydrate as a possible treatment for Parkinson’s disease , a large new double-blind, placebo-controlled trial found that this treatment does not improve clinical outcomes in patients with PD.
The new findings “do not support the use of creatine” in patients with early PD treated with background dopaminergic therapy, the study authors, with corresponding author Karl Kieburtz, MD, MPH, from the University of Rochester Center for Human Experimental Therapeutics, New York, conclude.
The trial was terminated early for futility on the basis of an interim analysis of 955 participants who had completed 5 years of follow-up.
The paper, prepared by the Working Group for the Neurological Disorders and Stroke Exploratory Trials of Parkinson Disease, which was created to promote discovery of potential therapies, is published in the February 10 issue of JAMA.
“Unfortunately, I think this study is truly the nail in the coffin for creatine in Parkinson’s disease,” commented Alberto Espay, MD, associate professor, neurology, Gardner Center for Parkinson’s Disease and Movement Disorders, University of Cincinnati, Ohio, and spokesperson for the International Parkinson and Movement Disorder Society, who was not part of the study group.
Nail in the Coffin?
Participants received creatine monohydrate or placebo.
When the trial was stopped at 5 years, 76% of the participants were still retained.
Dosage Issue?
What About Its Side Effects
Creatine is not associated with any adverse effects when used in a moderate amount. Several clinical trials have shown that it is well-tolerated and has a good safety record. When tested in Parkinsons patients, its intake didnt worsen the patients symptoms.
Disclaimer: The information shared here should not be taken as medical advice. The opinions presented here are not intended to treat any health conditions. For your specific medical problem, consult with your health care provider.
Recommended Reading: Parkinson’s And Extreme Fatigue
Affiliations Of Authors/writing Group For The Ninds Exploratory Trials In Parkinson Disease Investigators
University of Rochester, Rochester, New York University of Texas Health Science Center at Houston Medical University of South Carolina, Charleston National Institutes of Health, Bethesda, Maryland University of South Florida, Tampa Pacific Health Research and Education Institute, Honolulu, Hawaii University of California, San Francisco State University of New York Downstate Medical Center, Brooklyn University of Vermont, Burlington University of Kentucky, Lexington University of Michigan, Ann Arbor University of Maryland School of Medicine, Baltimore University of Pennsylvania, Philadelphia University of Calgary, Calgary, Alberta, Canada University of Texas Southwestern Medical Center, Dallas University of Southern California, Los Angeles Ochsner Medical Center, New Orleans, Louisiana University of Colorado Denver, Aurora The Parkinsons Institute and Clinical Center, Sunnyvale, California Johns Hopkins University, Baltimore, Maryland Georgia Regents University, Augusta Struthers Parkinsons Center, Golden Valley, Minnesota Thomas Jefferson University, Philadelphia, Pennsylvania Rush University Medical Center, Chicago, Illinois Beth Israel Deaconess Medical Center, Boston, Massachusetts Northwestern University, Chicago, Illinois University of Miami, Miami, Florida Brigham and Womens Hospital, Boston, Massachusetts .
Interim Efficacy Analysis Of Cohort 1
The interim analysis of cohort 1 determined that the mean of the summed ranks of the GST for placebo was 2360 and for creatine was 2414 . Higher summed ranks indicate worse outcomes. The GST, adjusted for site, yielded t1865.8 = 0.75 and did not exceed the OBrien-Fleming critical value of = .0027. There was no detected benefit or harm attributable to creatine at the time of LS-1 termination.
Table 2 reports the 95% CIs for each of the 5 components of the global score that make up the GST for cohort 1 at 5 years.
Read Also: What Is The Difference Between Huntington’s Disease And Parkinson’s
Us To Test Supplement Against Parkinson’s Disease
3 Min Read
WASHINGTON – U.S. government researchers launched a trial on Thursday testing creatine, a supplement sold to improve exercise performance, against Parkinsons disease.
Boxing legend Muhammad Ali pretends to punch actor Michael J.Fox before a Senate subcommittee hearing on Parkinson’s Disease on Capitol Hill in Washington in this May 22, 2002 file photo. U.S. government researchers launched a trial on Thursday testing creatine, a supplement sold to improve exercise performance, against Parkinson’s disease. REUTERS/William Philpott WP
Early research suggests creatine supplements might be able to help slow the progression of Parkinsons, an incurable brain disorder that can slowly but steadily paralyze patients.
The National Institute of Neurological Disorders and Stroke, one of the U.S. National Institutes of Health, is launching the trial as the first in a series of government-sponsored studies of new Parkinsons treatments.
Many clinical studies in the United States are sponsored by drug companies, which means that often only medicines they can make money on get tested.
The NINDS will recruit 1,720 people with early-stage Parkinsons disease across the United States and Canada. Patients and doctors alike will not know whether they are getting creatine or a placebo. The study is due to last three to five years.
The goal is to improve the quality of life for people with Parkinsons for a longer period of time than is possible with existing therapies.
Creatine For Parkinson’s Disease: Nutritional Supplement May Slow Progression Of Disease
- Date:
- NIH/National Institute of Neurological Disorders and Stroke
- Summary:
- The NIH National Institute of Neurological Disorders and Stroke is launching a large-scale clinical trial to learn if the nutritional supplement creatine can slow the progression of Parkinson’s disease . While creatine is not an approved therapy for PD or any other condition, it is widely thought to improve exercise performance. The potential benefit of creatine for PD was identified by Parkinson’s researchers through a new rapid method for screening potential compounds.
The NIH National Institute of Neurological Disorders and Stroke today is launching a large-scale clinical trial to learn if the nutritional supplement creatine can slow the progression of Parkinson’s disease . While creatine is not an approved therapy for PD or any other condition, it is widely thought to improve exercise performance. The potential benefit of creatine for PD was identified by Parkinson’s researchers through a new rapid method for screening potential compounds.
The double-blind, placebo-controlled, phase III study is one of the largest PD clinical trials to date. It will enroll 1720 people with early-stage PD at 51 medical centers in the United States and Canada.
“This study is an example of the Institute’s commitment to Parkinson’s research,” says NINDS director Story C. Landis, Ph.D. “We are trying to explore every possible option for reducing the burden of this disease.”
Story Source:
Read Also: Test To See If You Have Parkinson’s Disease
How About Studies In Human
Creatine supplementation has also been used in clinical trials performed in patients with Parkinsons disease. Unfortunately, the results were not promising in most cases.
In a follow-up study, creatine potential therapeutic effect was assessed in 1741 participants from 45 investigative sites in the USA and Canada. The participants were treated daily with 10 g creatine or placebo. Although the study was originally designed for a long term, after a planned interim analysis of 955 participants for 5 years, the investigators terminated the study trial. They reasoned that creatine consumption, when compared to placebo, for 5 years didnt have any beneficial effects in patients .
One meta-analysis study published in 2017 showed that creatine has no therapeutic effects against Parkinsons disease. In this study, results from five randomized controlled trials, conducted on 1339 participants, were selected. The study concluded that creatine consumption showed no improvement in patients quality of life and motor functions .
There are other studies which do not support the use of creatine in patients with Parkinsons disease . Only one study reported that it can improve upper body strength in patients .
Creatine And Parkinsons Disease Is There A Connection
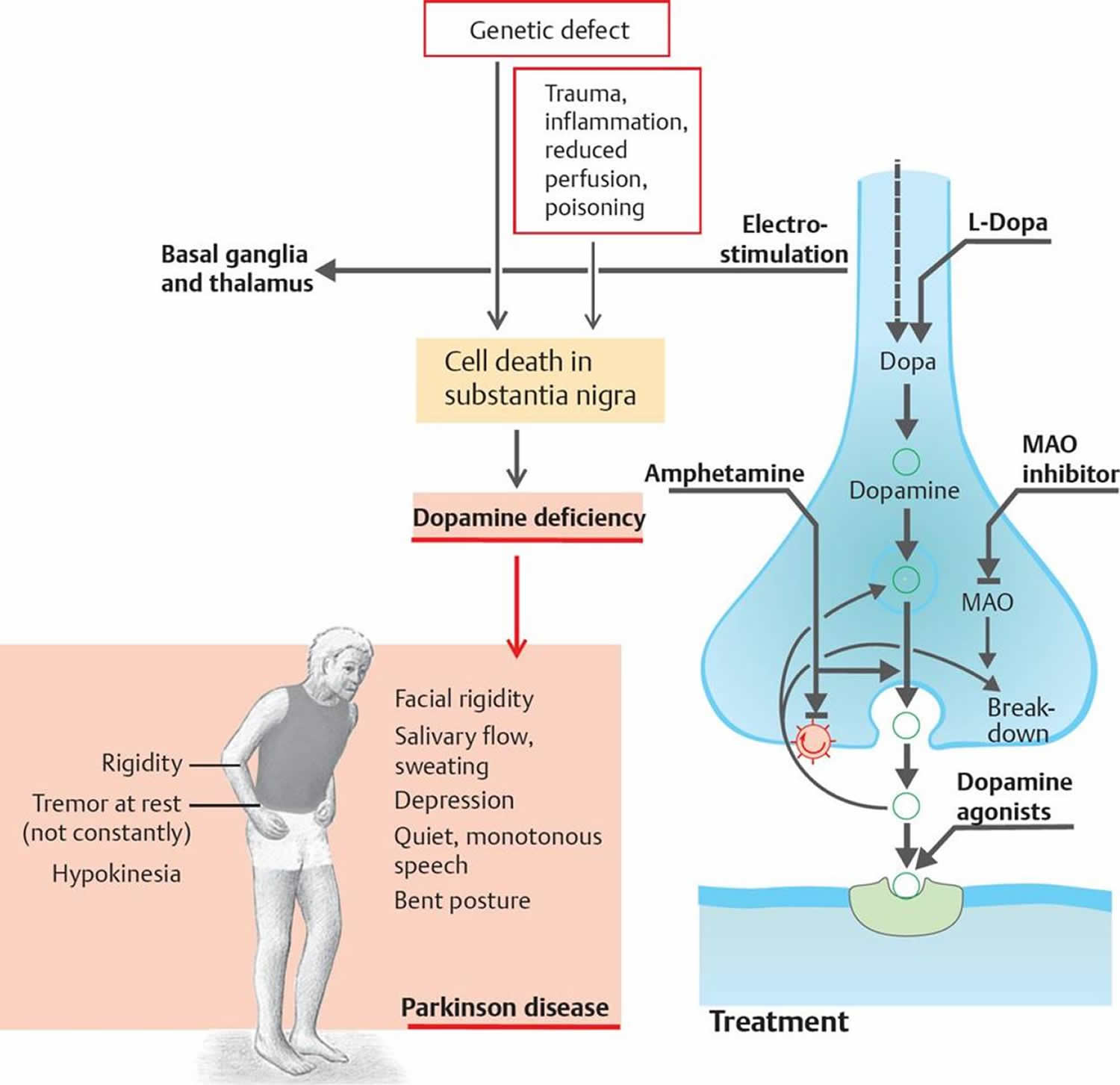
Parkinsons disease is a neurological condition that develops when the dopamine-producing cells of the brain are lost. Dopamine is a neurotransmitter that controls movement. When dopamine production ceased in the brain, the body show problems like shaking hands, slow movement, stiffness, and balance problem all these are classical signs of Parkinsons disease.
While the exact cause of the disease is still unknown, research shows that mitochondrial dysfunction is the key event in disease development . Mitochondria are the important organelles in the cell that generate energy in the form of ATP. Because of this function, mitochondria are also called the powerhouses of the cell.
Creatine helps mitochondria to keep them in a healthy state. It protects mitochondria from the insult of oxidative stress, which is linked to Parkinsons disease . Creatine has been shown to boost the activities of proteins that are crucial for mitochondrial quality and function .
Given that mitochondrial dysfunction is the key event in Parkinsons disease and that creatine is indispensable for mitochondrial health, it is plausible to think that there is a link between Parkinsonss disease and creatine. In fact, many researchers think that creatine may have the potential to modify the disease condition.
Read Also: Medications For Tremors In Parkinson’s