What Are The Symptoms Of Parkinson’s Disease
Parkinson’s DIsease can include a variety of symptoms that vary in severity and type amongst the affected population. Early signs of the condition can sometimes go unnoticed but as the disease progresses one can expect these symptoms:
- Difficulty speaking
- Difficulty writing
- Loss of automatic movements
- Slowed overall movement
- Muscle stiffness
Amneal Tests A New Formulation
AmnealPharmaceuticals plans to report Phase III safety results for IPX-203, a reformulation of the common generic PD treatment combination of carbidopa and levodopa that could reduce symptom fluctuations. The company said the Phase III, open-label extension study will have results available by the end of the second quarter of 2022.
CD/LD can lead to troughs and spikes of plasma levels that generate side-effects like dyskinesia, Kordower explains. A new extended-release version of CD/LD could smooth out these drops, he notes.
If approved, IPX-203 will join several other marketed reformulations of CD/LD. Amneals own extended-release capsule Rytary, Schwarz Pharmas orally disintegrating tablet Parcopa, and AbbVie’s enteral suspension Duopa all have FDA approval in PD. A GlobalData consensus forecasts pegs peak IPX-203 sales at $127 million in 2028.
In a separate, placebo-controlled Phase III trial , IPX-203 resulted in 0.53 more hours of ON time than immediate-release CD/LD after seven weeks . Earlier, a six-week Phase II trial of IPX-203 reported no serious treatment-emergent adverse events among the 26 patients enrolled. Experts say the long-term safety data will be key in determining IPX-203s place among CD/LD formulations.
What Are Mesenchymal Stem Cells
Stem cells are the body’s raw materials â cells from which all other cells with specialized functions are created. Mesenchymal stem cells are adult stem cells that have self-renewal, immunomodulatory, anti-inflammatory, signaling, and differentiation properties. Mesenchymal stem cells , self renewal capacity is characterized by their ability to divide and develop into multiple specialized cell types present in a specific tissue or organ.
Mesenchymal stem cells can be sourced from a variety of tissue including adipose tissue , bone marrow, umbilical cord tissue, blood, liver, dental pulp, and skin.
MSCs are widely used in the treatment of various diseases due to their self-renewable, differentiation, anti-inflammatory, and immunomodulatory properties. In-vitro and in-vivo studies have supported the understanding mechanisms, safety, and efficacy of MSC therapy in clinical applications.
Read Also: Phones For People With Parkinsons
The Promising Treatments In The 2022 Parkinsons Clinical Trials Pipeline
Posted:
As we know the Parkinsons research community is hard at work to find therapies and treatments to improve the quality of life of Canadians living with Parkinsons and ultimately find a cure. An article outlining the current clinical trials pipeline for Parkinsons was recently published in the Journal of Parkinsons Disease. Here are some of the key highlights. For a refresher on clinical trials and what happens in each phase, here is a helpful FAQ.
As of January 2022, there were nearly 150 Parkinsons therapies active in the clinical trial pipeline:
- 50 products in Phase I
- 65 products in Phase II
- 17 products in Phase III
Most of the drugs in trials are for managing the symptoms of Parkinsons . Developments in disease-modifying treatments the types of treatment that change the course of Parkinsons progression have been slow to emerge. However, in 2022, there are 54 disease-modifying treatments in trial phases, with three graduating to Phase III trials!
Below youll find a brief overview of whats happening in each phase of the trials.
Parkinsons Disease Psychosis Medication Approved
The FDA approved the first medication specified for the treatment of PD psychosis in May 2016. The drug, Nuplazid, may help relieve symptoms such as hallucinations and delusions experienced by the approximately 50 percent of Parkinsons patients with PD psychosis.
Nuplazid is the first and only FDA-approved therapy proven to control hallucinations and delusions in PD psychosis with no known adverse impacts on motor function. The drug was granted priority review and breakthrough therapy designation prior to its approval last spring. Breakthrough therapy designation is designed to expedite the development and review of drugs intended to treat a serious condition and where preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy.
The hallucinations and delusions experienced with Parkinsons disease can lead to thinking and emotions that are so impaired that the people experiencing them may not relate to loved ones well or take appropriate care of themselves, the FDA stated in its announcement of the drugs approval. The psychiatric disturbances of PD psychosis often are the result of standard Parkinsons drugs.
Recommended Reading: Best Beds For Parkinson’s Patients
Treatments In Phase Ii Trials
Another strategy in the therapeutic research space is drug repurposing. This is when an existing medication for one condition is repurposed to treat an entirely different condition. Working with repurposed medications comes with many advantages including understanding its general safety. Repurposing an existing medication, rather than starting from scratch, typically requires fewer tests for safety as the drug has already met these requirements. This can reduce costs and speed up the process through the clinical trial pipeline. It can also lead to faster approvals, getting much-needed treatments into the hands of people with Parkinsons as soon as possible. There are a total of 74 therapies in Phase II trials and 44% are repurposed medications.
One exciting takeaway from Phase II trials this year is the progress made with stem cell therapies. While there are nine stem cell therapies being explored in Phase I, two stem cell therapies graduated to Phase II trials this year! Moving into Phase II means these treatments are being administered to a larger group of people to monitor their effectiveness and further evaluate their safety.
Recommended Reading: What Is Treatment For Parkinsons Disease
Parkinsons Disease: A Hopeful Future
Parkinsons disease is the fastest growing neurological condition in the world. Between 1990 and 2015, the number of people with this disease doubled to over 6 million . This number is predicted to double again to 12 million by 2040, primarily because of an ageing population . In the UK, the lifetime risk of developing Parkinsons disease is 2.7%, with approximately 17,300 new diagnoses made every year in people aged 45 and above .
Parkinsons disease is a progressive, neurodegenerative disorder of the brain . It is characterised by the death of certain brain cells , particularly in an area of the brain called the substantia nigra . A high proportion of neurons within this part of the brain use a chemical called dopamine to transmit signals between themselves and throughout the brain these signals act to coordinate movement .
As the neurons in the substantia nigra die off, the amount of dopamine falls, which results in some of the major features of Parkinsons disease, such as slowness and stiffness of movement .
Adapted from International Society for Stem Cell Research 2022.
These symptoms increase in severity over time as more neurons are lost, and eventually patients may experience a resting tremor and have problems walking . Approximately 80% of patients also develop dementia within 20 years of disease onset .
Current treatment options target the symptoms, but not the cause
A new era: Targets for the Parkinsons disease medication pipeline
Lewy body accumulation
Recommended Reading: What Age Do You Develop Parkinson’s
Search For Parkinsons Disease
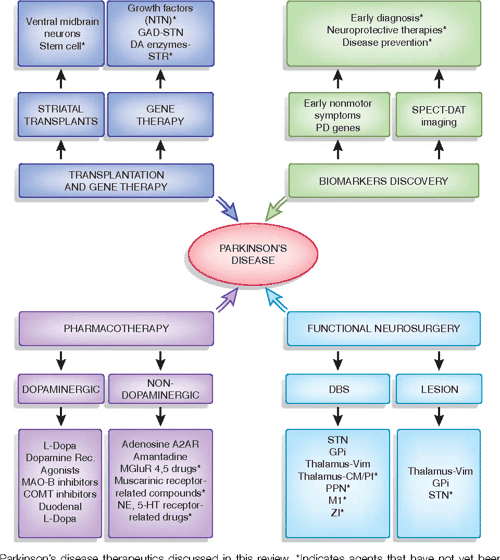
The ultimate therapeutic challenge remains. Every person who knows a patient with PD asks why there is not a treatment that prevents the motor manifestation of PD. Already-diagnosed PD patients and their families dream of a treatment that can slow down or even partially reverse the progression of this devastating disorder with all its motor and non-motor symptoms and complications in the advanced stage. These dreams may be fulfilled in the not-too-distant future.
Two therapeutic strategies are currently followed. The first is based on epidemiological findings and large clinical prospective trials reporting a correlation between a reduced occurrence or prevalence of PD and the consumption of compounds such as caffeine or nicotine . lists examples of these generic substances with a postulated disease-modifying potential for PD.
Important Points About The New Medications
With multiple new medications available for the treatment of PD, there is more hope than ever that Parkinsons symptoms can be successfully managed for many years. A few things to consider:
- For people whose symptoms are difficult to control, these new treatments are welcome additions to what was previously available and many people with PD have been using these new medications with significant benefit.
- On the other hand, many of the newly-approved medications have the same mechanisms of action as older medications so they are not breaking new ground in treating symptoms.
- In addition, for some people, the effect on symptoms may be mild or not substantial.
These caveats may mean that your physician has not suggested a medication change for you. It is also important to note that despite all the new medications, carbidopa/levodopa remains the most potent medication to treat the motor symptoms of PD.
If your doctor does choose to try one of the new options, there may be multiple paths that your doctor can take when contemplating a medication adjustment. Often trial and error is the only way to determine the best medication regimen for you, so you may need to practice some patience as you work together with your doctor to determine what works or doesnt work.
Read Also: What Tests Are Done To Diagnose Parkinson’s Disease
Aptinyx Focuses On Parkinsons Disease Cognitive Impairment
Aptinyx is amid a Phase II trial targeting one of the biggest unmet needs in Parkinson’s disease treatment: cognitive impairment and dementia.
In patients with PD, Lewy bodies and alpha-synuclein, which are implicated in the pathology of PD, can spread into the cortex, causing forms of cognitive impairment and dementia in a large percentage of patients, Kordower explains. Theres no current treatment, so any clinical trial that has some benefit is incredibly valuable, he says.
Aptinyxs NYX-458, which targets the N-methyl-D-aspartate receptor, has placebo-controlled Phase II results expected at the end of 2022 or early 2023. The trial lists eight primary endpoints, ranging from the incidence adverse events to reductions in scales of psychosis and suicidal ideation.
“There’s no current treatment, so any clinical trial that has some benefit is incredibly valuable.”
Jeffrey Kordower
Targeting NMDA is a good start, says Dr David Eidelberg, neurologist at the Feinstein Institutes for Medical Research. However, showing reduction in cognitive impairment may be more difficult in PD than in Alzheimers disease, where changes are typically more pronounced, he notes.
Nevertheless, experts are encouraged that a trial is taking aim at this substantial unmet need. According to GlobalData consensus forecasts, NYX-458 has expected peak sales of $323 million in 2028.
Cannabinoid Mixtures Ease Parkinsons Motor Symptoms In Zebrafish
According to InMeds press release, two cannabinoid analogs have shown promising results for neurodegenerative diseases, specifically, in promoting the growth of neurites, cell body extensions that nerve cells normally use to communicate with each other.
Our early studies are showing promising neuroprotective effects as well as neurite outgrowth, signifying the potential to enhance neuronal function that may be important in the treatment of neurodegenerative diseases, said Eric Hsu, PhD, senior vice president of preclinical Research & Development at InMed.
The company will be conducting studies in in vivo models of neurodegenerative diseases.
Our early studies are showing promising neuroprotective effects as well as neurite outgrowth, signifying the potential to enhance neuronal function that may be important in the treatment of neurodegenerative diseases.
We are pleased that our efforts have led to the identification of two cannabinoid analog candidates to advance to in vivo studies, Hsu said.
The first preclinical efficacy results are expected by June 2023. The research will be conducted in collaboration with Ujendra Kumar, PhD, professor of Pharmaceuticals Sciences at the University of British Columbia , Vancouver, Canada.
Our team will continue this important research in neurodegenerative diseases under the NSERC Alliance grant, Hsu said.
Recommended Reading: Differences Between Parkinson’s And Alzheimer’s
Reasons Parkinsons Disease Can Occur
When a loved one is living with Parkinsons, its natural to wonder what caused the disease to take root. Although most cases of Parkisons have an unknown cause, a very small percent of cases can be hereditary . Some studies have linked long-term exposure to pesticides, including one called paraquat, with an increased likelihood of the development of Alzheimers and Parkinsons disease.
The pesticide was first introduced in the United States in the 1950s and is still used commonly by licensed users today. If you think that your loved one who has been diagnosed with Parkinsons may have been exposed to paraquat, its important that you work with a lawyer to learn whether you may be eligible for compensation. Caring for a loved one with Parkinsons can be costly and time-consuming, especially in the later stages when your loved one requires around-the-clock care. Financial compensation can make it easier to provide your loved one with the care they deserve throughout the progression of the disease.
Were here to help you decide what to do next after you find out that paraquat exposure may be responsible for the development of Parkinsons disease in your loved one. Reach out to us today for a free case review.
The Motor Network In Parkinsons Disease And Dystonia: Mechanisms Of Therapy

open to eligible people ages 21-75
This is an exploratory pilot study to identify neural correlates of specific motor signs in Parkinsons disease and dystonia, using a novel totally implanted neural interface that senses brain activity as well as delivering therapeutic stimulation. Parkinsons disease and isolated dystonia patients will be implanted unilaterally or bilaterally with a totally internalized bidirectional neural interface, Medtronic Summit RC+S. This study includes three populations: ten PD patients undergoing deep brain stimulation in the subthalamic nucleus , ten PD patients with a globus pallidus target and five dystonia patients. All groups will test a variety of strategies for feedback-controlled deep brain stimulation, and all patients will undergo a blinded, small pilot clinical trial of closed-loop stimulation for thirty days.
San Francisco, California
Recommended Reading: What Is An Essential Tremor Parkinson
Also Check: Latest Developments In Parkinson’s Treatment
The History Of Parkinsons
Parkinsons disease was first defined as a shaking palsy in 1817 by James Parkinson. Half a century later, in 1872, the Parisian neurologist Jean-Martin Charcot coined the term Parkinsons disease.
Though Parkinson was the first to describe the disease in modern medicine, Charcot and his colleagues revolutionised treatments in the mid-19th century.. Parkinson was a proponent of blood-letting from the neck, in a bid to siphon off inflammatory pathogens and prevent them from reaching the brain. But Charcot and his colleagues favoured pharmaceutical approaches centred around anticholinergic drugs, which block the action of a neurotransmitter called acetylcholine. Anticholinergics are still in use today.
Around the same time, a host of other treatments were being explored at a hospital in Paris. Hyoscyamine, a plant-derived medication, was put in bread and fed to patients. Other medications, such as a derivative of quinine, were mixed with a syrup of orange rinds.
Charcot also claimed to see the symptoms of patients with Parkinsons improving when travelling by train and horse-carriage. He became a proponent of vibration therapy, where patients bodies and heads were shaken vigorously by a rigged motor.
Component #2 A Neuroprotective Agent
Once a drug or a treatment has been determined to slow down the progression of Parkinsons, it will be necessary to protect the remaining cells and provide a nurturing environment for the third part of the cure .
Neuroprotection is the area of research that has had the most attention over the years. Drug companies have employed vast resources in this area in the hope of discovering a treatment which will work across conditions , and thus provide them with tremendous profits. Unfortunately, conditions of the brain have proven to be a lot more complicated than first perceived and cross-condition therapies seem unlikely as we move towards greater stratification and personalisation of disease and treatment, respectively.
But there has been the hint of a potential neuroprotective effect in one class of drugs for Parkinsons: GLP-1R agonists.
Neuroprotective approach: GLP-1R agonists
Exenatide is a glucagon like peptide-1 receptor agonist. This is a class of drug that has traditionally been used for treating diabetes, but has recently been repurposed for Parkinsons.
After multiple studies suggested neuroprotective properties in models of Parkinsons, a clinical trial program was intiated, and in 2017, a Phase II Exenatide trial reported the stablisation of Parkinsons motor features over the course of the 48 week trial .
Reduction in motor scores in Exenatide group. Source: Lancet
In late 2019, we saw the initiation of a Phase III clinical trial for .
Read Also: Does Cannabis Help Parkinson’s
Irlab Celon Target Parkinsons Disease Motor Symptoms
While levodopa can reduce PD symptoms by increasing dopamine levels in the brain, prolonged use can cause increased bouts of dyskinesia during OFF time when the drug has reduced effects. IRLAB Therapeutics and Celon Pharma have Phase II trials aiming to reduce levodopa-induced dyskinesia using two different approaches.
IRLABs mesdopetam, which targets the dopamine D3 receptor, has placebo-controlled Phase II trial results expected the second half of this year. Mesdopetam has estimated peak sales of $226 million in 2026, according to GlobalDatas consensus forecast. GlobalData is the parent company of Clinical Trials Arena.
As a primary endpoint, the Phase II mesdopetam trial assesses change in ON time, defined as hours in the day where patients experience the positive effects of levodopa without the negative effects of dyskinesia. Before and after 12 weeks of treatment, patients record their hours of ON time over the course of 24 hours.
Meanwhile, Celons CPL500036, which targets phosphodiesterase 10A , also has placebo-controlled Phase II results expected this year. The four-week study uses a primary endpoint of the Unified Dyskinesia Rating Scale , which measures the severity of dyskinesia side effects.
Of the two primary endpoints, change in ON time is a better measure than the UDyRS, Kordower says. My understanding is that patients would much rather have less time spent in dyskinesias than decreased magnitude of dyskinesia, he explains.