Where Do Stem Cells Come From
Various types of stem cells are found at different stages of human development and in different parts of the body, all of which are of interest to researchers.
Embryonic stem cells: these stem cells are found in fertilised eggs that are only a few days old. These are the only cells that can develop into any type of cell within the body. The abundance of stem cells decreases as the embryo grows and stem cells become specialised cell types that form parts of our body.
Whilst embryonic stem cells are very promising as a treatment for Parkinsons, they also have the risk of uncontrolled growth in the body which could lead to the formation of tumours. Much more research is needed in order for scientists to understand how stem cells work and how they may be used to produce treatments for Parkinsons and many other medical conditions.
Adult stem cells: although adult stem cells exist, they are found in quite small numbers in certain parts of the body and do not multiply quickly. Adult stem cells have been used to treat some conditions for example bone marrow transplants to treat leukaemia. However, it is not currently possible to change adult stem cells into nerve cells suitable as a treatment for Parkinsons.
Bone marrow: these stem cells are found in our bone marrow and bones.
Stem Cell Treatments In The Future Of Parkinsons Disease Management
Although there are a number of challenges brought about with stem cell-based treatments for PD, it seems probable that these treatments will progress to the clinic in the short- to medium-term future. While development of optimized products has been necessarily slow and iterative, the field is now asking questions about how these treatments can be scaled and deliveredthis demonstrates the progress that has been made with these approaches.
As has been discussed, the purpose of stem cell treatments is predominantly to treat the motor symptoms of PD. They will not have any disease-modifying effect and will not treat the major non-motor symptoms which can be particularly disabling in some patients. While these techniques can form one arm of the future of PD treatment, they will likely be combined with other novel treatments targeting alpha-synuclein pathology . It may be possible for stem cell-based regenerative therapies to be employed to restore the function of dopaminergic neurons that have already been lost, while novel disease-modifying drugs could be used to prevent ongoing neuronal death.
Pure Substantia Nigra Mda Neuron
Recently, single cell gene profiling has been used to define subtype compositions during mouse and human midbrain development . Such technology extended the previous molecular definition of mDA neurons and enabled to further divide SN and VTA region into seven distinct molecular clusters . However, to what extent these molecular findings can be translated into hPSC-derived mDA neuron development remains unexplored. This is illustrated when profiling of hPSC-derived mDA neurons in La Manno et al., which seem to recapitulate key stages of in vivo ventral midbrain development. However, those cell preparations expressed many poorly defined radial glial and neuroblast markers and differed from the in vivo phenotypes in gene expression . Additionally, ALDH1A1 was not expressed in any of those PSC-derived mDA cells. This result may be due to the in vitro culture environment that does not fully support mDA neuron development or lack of proper induction of certain subtype-specific genes. In either case, those results indicate that there is considerable room for further improvements in mDA neuron derivation and maturation strategies.
Dont Miss: Development Of Parkinsons Disease
Recommended Reading: Fitflop Shoes For Parkinson’s
Some Key Questions To Ask About Unproven Therapies Being Offered Abroad
Have pre-clinical studies been published, peer-reviewed and repeated by other experts?Do the providers have independent committee approval, e.g., Institutional or Ethics Review Board, to ensure risks are as low and worth any potential benefits, and that patient rights are being protected?Do the providers have approval for the safe conduct of clinical trials or medical use of a product for PD from a national or regional regulatory agency, e.g., FDA or the European Medicines Agency?What are the alternative treatment options for my condition?If I have this treatment, can I participate in otherclinical trials or have other interventional treatment options?What compensation am I entitled to if I am injured as a result of participating in this therapy?What are the total costs of the treatment and what does this include?What would be the costs of emergency treatment, if needed, should a complication arise?
Benefits And Risks Of Clinical Trials
Participating in a clinical trial can be a rewarding experience. Consider the following benefits when deciding if you should join a clinical trial:
- You will have access to leading healthcare professionals, cutting-edge new treatments and high standards of care.
- Joining a clinical trial can increase your knowledge and understanding of your disease.
- People who take part in clinical trials are contributing to science that may benefit themselves and others. The medications that you take now are available only because people before you have volunteered in clinical trials.
Although every effort is made to ensure that clinical trials are as safe as possible, clinical trials that test new therapies are experiments and can involve risks.
Here are some of the risks to consider:
- There may be undesirable side effects to the treatment. A health professional will explain possible risks and side effects during the informed consent process.
- The treatment may not be effective for the participant.
Recommended Reading: Parkinson Bicycle Cleveland Clinic
What Are The Early Signs Of Parkinsons Disease
Early symptoms and possible warning signs of Parkinsons disease are: Writing becomes difficult and font is getting involuntarily smaller One sided tremor or shaking Involuntarily index finger and thumb rolling Dizziness or fainting Change of facial expression known as mask face Soft or lower voice then usual Sleep problems usually accompanied with sudden movements Walking and otherwise subconscious movements only seem possible when actively imitating them Hunching over or stooping
Dont Miss: Cleveland Clinic Parkinsons Bicycle Study 2017
The Promising Treatments In The 2022 Parkinsons Clinical Trials Pipeline
Posted:
As we know the Parkinsons research community is hard at work to find therapies and treatments to improve the quality of life of Canadians living with Parkinsons and ultimately find a cure. An article outlining the current clinical trials pipeline for Parkinsons was recently published in the Journal of Parkinsons Disease. Here are some of the key highlights. For a refresher on clinical trials and what happens in each phase, here is a helpful FAQ.
As of January 2022, there were nearly 150 Parkinsons therapies active in the clinical trial pipeline:
- 50 products in Phase I
- 65 products in Phase II
- 17 products in Phase III
Most of the drugs in trials are for managing the symptoms of Parkinsons . Developments in disease-modifying treatments the types of treatment that change the course of Parkinsons progression have been slow to emerge. However, in 2022, there are 54 disease-modifying treatments in trial phases, with three graduating to Phase III trials!
Below youll find a brief overview of whats happening in each phase of the trials.
You May Like: On And Off Phenomenon
Etiology And Risk Factors
Parkinsonian symptoms can arise from either the neuropathologic condition of PD or other forms of parkinsonism. For neuropathologic PD, about 90% of cases are sporadic, with no clear etiology an additional 10% have a genetic origin, and at least 11 different linkages with 6 gene mutations have been identified Genetic forms of PD are seen more frequently in young-onset PD. A combination of environmental factors or toxins, genetic susceptibility, and the aging process may account for many sporadic cases Secondary forms of parkinsonism can be caused by medications, the sequelae of central nervous system infection, toxins, or vascular/metabolic disorders . The only proven risk factor for PD is advancing age . Other environmental or lifestyle risk factors associated with development of PD are rural living, exposure to pesticides and herbicides, well-water drinking, and working with solvents . However, none of these factors unequivocally has been demonstrated to cause iPD .
Figure 1: Etiology of Parkinsons Disease . View Figure 1
Recommended Reading: Sam Waterston Tremor
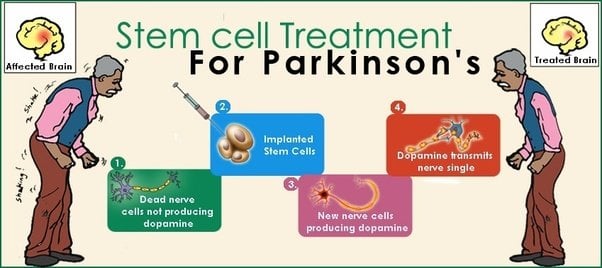
What Can Stem Cell Therapy Do For Parkinsons Disease
Stem cells have been shown to improve the symptoms of patients with Parkinsons Disease. Stem cells from fat may be able to differentiate into new, healthy, dopamine-creating neurons the same cells that are damaged by the disease. Stem cell therapy may also reduce inflammation caused by the condition. See our blogs What are Stem Cells? and Understanding Adipose-Derived Stem Cells to learn more about how stem cells are obtained from fat. Many patients experience improvements in their condition and a reduction in Parkinsons-related symptoms.
You May Like: Similar To Parkinsons
Recommended Reading: Parkinson’s Hallucinations Commercial
Paving The Way To New Horizons
Two years after the study began, time seems to be on Kim and Lopezs side. The cells are still alive, and the study has shown favorable outcomes. However, the one-person model was not a traditional blind study. Lopez knew he was receiving the therapy. Did his mindset create a placebo effect? Why only one patient?
Since our approach is personalized therapy, even the big scale trial should start from an individual patient, Kim said. So, we thought that we better start with a single patient and gain all experiences and information to move on.
The one-person study introducing personalized therapy seems to have paved the way for additional research. However, Kim noted that a double-blind study is needed to conclude its safety and efficacy.
I am grateful to Lopez who funded the trial and accepted the risks. He and Kim, together with all patients and doctors that participate in Parkinsons research, have the people with Parkinsons as their catalyst to keep pressing forward. Sometimes its hard to see the forest through the trees, but the goal is the same: a therapy for us all. Ultimately, that is what is important even if it is one person at a time.
What Are The Stages Of Parkinsons Disease
- Parkinson Stage 1: Only mild symptoms occur in facial expressions or slight tremor. However, the daily life is only minimally affected and only one sided.
- Parkinson Stage 2: At this stage symptoms are getting worse and affect both sides. The tremor and waling problems are getting worse. Often this is the stage where L-Dopamine is employed.
- Parkinson Stage 3: Loss of balance and slowed movements characterize this stage. Living independently is still possible but symptoms significantly impair daily activities.
- Parkinson Stage 4: The Symptoms are severely limiting every activity, especially walking. Movements are still possible but may require aid. Usually living alone is not possible at this stage any more.
- Parkinson Stage 5: The final and most debilitating stage, where stiffness in legs makes movement impossible. The person requires full time nursing and is bound to the wheelchair. Usually many non-motoric symptoms set like delusions, hallucinations and depressions.
You May Like: Can Lead Poisoning Cause Parkinsons
Don’t Miss: On Off Phenomenon
What Diseases And Conditions Resemble Parkinsons Disease
PD is the most common form of parkinsonism, in which disorders of other causes produce features and symptoms that closely resemble Parkinsons disease. Many disorders can cause symptoms similar to those of PD, including:
Several diseases, including MSA, CBD, and PSP, are sometimes referred to as Parkinsons-plus diseases because they have the symptoms of PD plus additional features.
In very rare cases, parkinsonian symptoms may appear in people before the age of 20. This condition is called juvenile parkinsonism. It often begins with dystonia and bradykinesia, and the symptoms often improve with levodopa medication.
Ipscs As Disease Model For Parkinsons Disease
The contribution of pharmacogenomics has been heightened through the use of patient-specific iPSC lines and genetic engineering technology to manipulate them. Ever since Yamanakas discovery in 2007 that a handful of transcription factors can reprogram cellular differentiation, iPSCs have been utilized extensively in the study of neurodegenerative disease to direct patient-specific cell fate . While still limited in scope, iPSCs are currently the most robust and phenotypically similar model for PD . Mutations of consequence can now be captured in iPSC lines and directed by small molecules to a DAn fate in PD modelsall within a dish. Displayed openly, the real-time cellular effects of mutation can be physically observed and studied in tandem with control lines to limit genetic background effects of the affected individual similarly, effects of oxidative stress common to PD can also be quantified with broad clinical applications for drug screening without human side-effects. Not surprisingly, it remains difficult to physically confirm the mechanisms of neurodegeneration and neuroprotection implicated by iPSC research as patients neurons are hidden deep within the brain. These effects similarly cannot be perfectly translated into the cellular environment of PD due to some epigenetic effects of aging eliminated in reprogramming protocols.
You May Like: Does Parkinsons Cause Swelling
You May Like: Parkinson Silverware
Stem Cells Have More Variety Than Id Thought
Kim referred to this particular study as very unusual and exceptional because it was the first trial of its kind. It reported successfully using Lopezs own skin to create induced pluripotent stem cells, which are cells that are reprogrammed to behave like stem cells. Those cells, which are autologous, were then transplanted into the Parkinsons patients brain. This is different from embryonic stem cells , which is an allogeneic approach.
OK, what do autologous and allogeneic mean? Allogeneic stem cells are not taken from ones own body, while autologous stem cells are created from ones own body.
Kim explained: There are different approaches, such as fetal cells and embryonic stem cells. These approaches could be complementary and not exclusive. For instance, the ESC-based approach may be more economic and fast because the cells can be available for many patients. However, these cells are allogeneic and need immunosuppression. In contrast, autologous cell transplantation does not need immunosuppression but will take more time and budget. Once FDA approval is obtained, these different approaches can be compared with each other.
First In The Nation Fda
A Phase II clinical trial to assess mesenchymal adult stem cells as a disease-modifying therapy for Parkinson’s disease has been launched at The University of Texas Health Science Center at Houston .
“Studies have shown mesenchymal stem cells can migrate to the sites of injury and respond to the environment by secreting several anti-inflammatory and growth factor molecules that can restore tissue equilibrium and disrupt neuronal death,” said Mya C. Schiess, MD, professor in the Department of Neurology and director and founder of the movement disorder subspecialty clinic and fellowship program of McGovern Medical School at UTHealth. “The stem cells interact directly with the immune cells, leading to an anti-inflammatory state that allows a restorative process to take place.”
Safety and tolerability results, assessed on a previous trial, were recently published in the journal Movement Disorders. The Phase I study showed that there were no serious adverse reactions related to the stem cell infusion and no immunological reactions to the cells, which come from the bone marrow of a healthy adult donor. The study enrolled 20 patients with mild to moderate disease, who were infused with one of four different dosages and monitored for a year. Additionally, researchers reported a reduction in peripheral inflammatory markers and a reduction in motor symptoms.
For media inquiries: 713-500-3030
Also Check: Prayers For Parkinson’s Disease
Fda Authorizes Simultaneous Stem Cell Trials For Parkinsons
SUGAR LAND, Texas—-Houston areanon-profit research organizationHope Biosciences Stem Cell Research Foundation has received Food and Drug Administration authorization for a Phase II clinical trial to assess the efficacy and safety of multiple intravenous infusions of allogeneic adipose-derived mesenchymal stem cells in improving activities of daily living and quality of life in subjects with Parkinsons Disease.
This 60-patient trial is the second FDA clinical trial authorization in Parkinsons for HBSCRF, who already has a 24-patient double-blind placebo trial underway utilizing autologous adipose-derived mesenchymal stem cells. HBSCRF will become the first research organization in the U.S. to conduct simultaneous trials examining effects of administering the patients own cells and donor cells on the same disease condition.
Participants in the Parkinsons trial can be male or female, must be between 45-80 years of age, and must have been diagnosed with mild-to-moderate Parkinsons at least two years prior to commencement. There is no cost to participate.
For more trial information, please call 346-900-0340.
Phase Iia Randomized Placebo Controlled Trial: Mesenchymal Stem Cells As A Disease
| The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government. Read our disclaimer for details. |
| Recruitment Status : Active, not recruitingFirst Posted : August 10, 2020Last Update Posted : February 10, 2022 |
- Study Details
| Drug: MSC+placeboDrug: MSCDrug: Placebo | Phase 2 |
| Study Type : | |
| Triple | |
| Primary Purpose: | Treatment |
| Official Title: | A Randomized, Double-blind, Placebo-controlled Trial of Allogeneic Bone Marrow-derived Mesenchymal Stem Cells as a Disease-modifying Therapy for Idiopathic Parkinson’s Disease |
| Actual Study Start Date : |
| 2 treatment doses + 1 placebo 3 months apart | Drug: MSC+placebo2 infusions of 10 X 10^6 MSC/kg and 1 placebo every 3 months.Other Name: allogeneic mesenchymal stem cell or similar placebo |
| Experimental: MSC3 treatment doses 3 months apart | Drug: MSC3 infusions of 10 X 10^6 MSC/kg every 3 months.Other Name: allogeneic mesenchymal stem cell |
| Placebo Comparator: Placebo3 placebo doses 3 months apart | Drug: Placebo3 infusions of placebo every 3 months. Placebo will be identical to the investigational product but will not contain MSCs.Other Name: Similar placebo |
Also Check: Parkinson’s Double Vision