How The Prism Parkinsons Research Study Might Change The Way We Treat Parkinsons
Three times in history, the stars aligned to create an environment in which a Parkinsons breakthrough therapy was possible.
The first was in 1960, when scientists first realized that dopamine was linked to Parkinsons a realization that helped researchers discover how levodopa could treat symptoms.
The second was in the 1990s, when an understanding of the connectivity of motor control led to the realization that deep brain stimulation could provide additional symptom control.
The third time might just be now.
Researchers are understanding better than ever the molecular pathogenesis of Parkinsons. With this more thorough understanding, scientists around the world are working to develop realistic candidates to slow, stop, or even reverse the progression of Parkinsons.
Exenatide And The Treatment Of Patients With Parkinsons Disease
1Sobell Department of Motor Neuroscience, UCL Institute of Neurology, London, United Kingdom. 2Department of Nuclear Medicine, University College London Hospitals NHS Trust, London, United Kingdom. 3Reta Lila Weston Laboratories, London, United Kingdom. 4UCL School of Pharmacy, London, United Kingdom. 5Cure Parkinsons Trust, St. Botolphs, London, United Kingdom.
Address correspondence to: Thomas Foltynie, Box 146, National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, United Kingdom. Phone: 0203.448.8726 Fax: 0203.448.0142 E-mail: .
Find articles byAviles-Olmos, I.in:JCI |PubMed |
1Sobell Department of Motor Neuroscience, UCL Institute of Neurology, London, United Kingdom. 2Department of Nuclear Medicine, University College London Hospitals NHS Trust, London, United Kingdom. 3Reta Lila Weston Laboratories, London, United Kingdom. 4UCL School of Pharmacy, London, United Kingdom. 5Cure Parkinsons Trust, St. Botolphs, London, United Kingdom.
Address correspondence to: Thomas Foltynie, Box 146, National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, United Kingdom. Phone: 0203.448.8726 Fax: 0203.448.0142 E-mail: .
Find articles byDickson, J.in:JCI |PubMed |
Address correspondence to: Thomas Foltynie, Box 146, National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, United Kingdom. Phone: 0203.448.8726 Fax: 0203.448.0142 E-mail: .
Find articles byKefalopoulou, Z.in:JCI |PubMed |
J Clin Invest.
Why Was The Practically Defined Off Used As The Primary Outcome
During the first years of PD, most patients experience a honeymoon period during which many of their symptoms can be dramatically improved by the use of dopaminergic drugs. As the disease advances, motor fluctuations can occur, typically necessitating adjustment of the dose and frequency of L-dopa administration. With this approach, patients best functional performance can remain stable for several years despite underlying progression of disease. During the later stages of disease, L-dopa refractory symptoms emerge and these can be measurable despite optimal therapy.
The Exenatide-PD trial recruited patients that were on doses of pharmacological therapy that had been kept relatively stable, but who reported that they had experienced periods of suboptimal symptom control signaling the onset of motor fluctuations. This allowed a window to estimate the underlying severity of PD by performing assessments in the widely adopted Practically defined OFF medication state, i.e., after an overnight period free of all PD medication. The assessments were performed at the same time in the morning and each patient had stopped his or her medication the night before, or at least 24 hours beforehand if taking a long acting dopamine agonist. This consistent timing of assessment in a drug free period represents an informative way to measure PD severity in a manner that is less affected by changes in dopaminergic drugs.
Also Check: Judy Woodruff Health Problems
Interested In Taking Part
This trial is not currently seeking participants but you can stay in touch with the study by keeping an eye on their web page here.
There are currently many Parkinsons research studies underway in the UK that do need participants including clinical trials testing potential new therapies.
Find studies to suit you using our Take Part Hub.
What About Other Aspects Of The Condition
Throughout the trial, every aspect of Parkinsons was carefully recorded through a huge range of assessments, including:
- quality of life
- changes in mood, memory and thinking
- comprehensive assessments of mobility while participants were on their regular Parkinsons medication
One of the most puzzling things about these results is that there was no improvement in the exenatide group on any of these other assessments compared to the placebo group.
This is particularly surprising considering the results from the 2013 trial, which showed that exenatide improved performance on both movement assessments and cognitive tests that assess memory and thinking.
Recommended Reading: Parkinson’s Bike Therapy
Adaptive Deep Brain Stimulation To Improve Motor And Gait Functions In Parkinson’s Disease
Sorry, accepting new patients by invitation only
This is a single-center phase I clinical study aiming to improve gait functions in patients with Parkinson’s disease by using adaptive neurostimulation to the pallidum. The investigators will use a bidirectional deep brain stimulation device with sensing and stimulation capabilities to 1) decode the physiological signatures of gait and gait adaptation by recording neural activities from the motor cortical areas and the globus pallidus during natural walking and a gait adaptation task, and 2) develop an adaptive deep brain stimulation paradigm to selectively stimulate the pallidum during different phases of the gait cycle and measure improvements in gait parameters. This is the first exploration of network dynamics of gait in PD using chronically implanted cortical and subcortical electrodes. In addition to providing insights into a fundamental process, the proposed therapy will deliver personalized neurostimulation based on individual physiological biomarkers to enhance locomotor skills in patients with PD. Ten patients with idiopathic Parkinson’s disease undergoing evaluation for DBS implantation will be enrolled in this single treatment arm study.
San Francisco, California
Following Encouraging Signs In Recent Phase 2 Studies A Large Uk
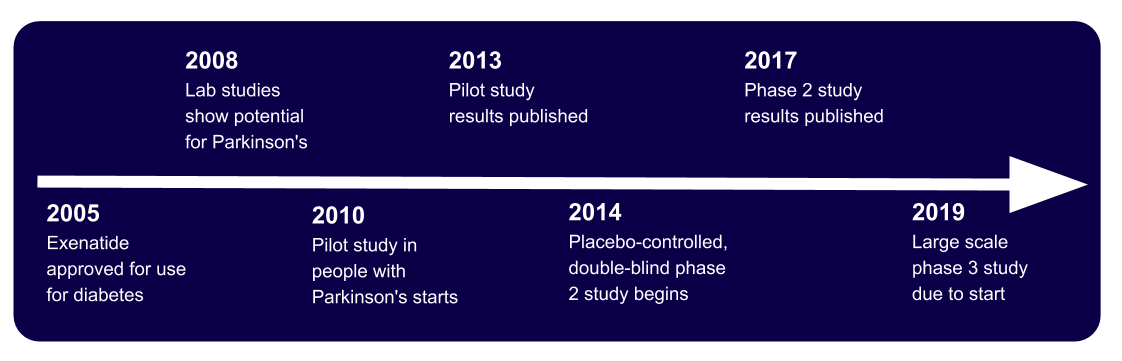
For the last decade researchers have been investigating whether exenatide an injectable drug that is used to treat type 2 diabetes could be repurposed to treat people with Parkinsons. This has painstaking work has led to a definitive phase 3 clinical trial that will test whether this treatment can slow the course of Parkinsons, something no currently available drug can do.
If the results are positive it could lead to the first completely new treatment for Parkinsons in years.
You May Like: Judy Woodruff Parkinson’s
As Several Big Pharma Companies Drop Parkinsons Projects Attention Turns To Gene Therapies And Repurposing Diabetes Drugs
Parkinsons disease research has ended in numerous dead ends despite substantial efforts over many years. Recently, Biogen and Sanofi scrapped their Parkinsons candidates, cipanemab and venglustat respectively, owing to lack of efficacy, and a disease-modifying therapy has yet to materialise.
But the push to find drugs that help beyond reducing symptoms continues, and Evaluate Vantage has delved into the pipeline of projects in active late-stage clinical trials. This year is shaping up to be crucial for the field, with 10 studies expected to yield data or to complete in 2021.
One target that crops up multiple times is GLP-1 this approach, traditionally employed in type 2 diabetes, is also being tested in Alzheimers. Among other avenues of research, it is hoped that gene therapy could offer a one-time cure for Parkinsons.
Repurposing
Research has suggested that GLP-1 agonists have neuroprotective benefits, and several trials of marketed diabetes drugs, as well as new GLP-1-targeting projects, are under way in Parkinsons. Some of these studies are investigator sponsored, including the most advanced, a UCL-run phase III trial of Astrazenecas Bydureon called Exenatide-PD3.
In the meantime, data are expected from several phase II studies of GLP-1 agonists, including a trial of Novo Nordisk’s Victoza, being run by Cedars-Sinai Medical in collaboration with the Danish company and The Cure Parkinson’s Trust. That study is set to complete in September.
Gene therapies
What Further Trial Evidence Is Needed
To provide irrefutable evidence that exenatide confers a therapeutic advantage to patients with PD requires a larger multi-site replication study. Given that most patients do not spend the majority of their time in the OFF-medication state, identifying a meaningful advantage will require a comparison of the severity of PD symptoms during optimal dopaminergic replacement therapy. This will require long term follow up to evaluate the emergence of dopa refractory motor and non-motor symptoms. This type of study is called a Long term simple design and is expensive to perform because of its long duration and the need to include large numbers of patients to compensate for potential dropout of some trial participants. Inclusion of an additional OFF medication assessment also adds to the complexity and the expense. However, given that all patients will receive optimal dopaminergic replacement therapy, dropout will hopefully be minimized. This type of study will answer the fundamental question whether PD patients are indeed advantaged in terms of their quality of life and functional ability when given exenatide, but would not necessarily reveal an underlying mechanism of action.
Recommended Reading: Fitflop Shoes For Parkinson’s
A Study To Evaluate The Efficacy Of Prasinezumab In Participants With Early Parkinson’s Disease
Sorry, in progress, not accepting new patients
This multicenter, randomized, double-blind, placebo-controlled, Phase 2 study will evaluate the efficacy of intravenous prasinezumab versus placebo over 52 weeks in participants with early Parkinson’s Disease who are untreated or treated with monoamine oxidase B inhibitors since baseline. The study will consist of three parts: a 52-week, double-blind, placebo-controlled treatment period after which eligible participants will continue into an all-participants-on-treatment blinded dose extension for an additional 52 weeks . Participants who complete Part 2 will be offered participation in Part 3 open-label extension for an additional 260 weeks.
San Francisco, California
Why Was The Trial So Short And With So Few Participants
The costs for conducting this trial were generously supported by the Michael J. Fox Foundation, except for the provision of the trial drug and placebo which were provided by first Bristol Myers Squibb and later following an acquisition by Astra Zeneca. Given that the patent protection for exenatide was due to expire at the end of 2016, the commercial appeal of developing exenatide for neurodegenerative diseases was low already when the trial was set up in 2013. Therefore, commercial support was limited to the supply of drug and placebo for 48 weeks only, in a maximum of 60 participants. A formal power calculation was performed which identified that this number of patients had 90% power to detect a difference of 5.8 MDS UPDRS points between the 2 groups .
Recommended Reading: On Off Phenomenon
Learn More About The Prism Parkinsons Research Study
Treatments that reverse, stop, or slow down the progressive loss of neuron function in people with Parkinsons do not currently exist. The PRISM Parkinsons Research Study is evaluating an investigational drug to determine if it might slow down the progression of Parkinsons. By participating in this study, you could help advance the development of treatment options. Enrollment will close soon. To learn more about the study and see if you might qualify for enrollment, .
This post was written by the Davis Phinney Foundation.
This post is sponsored by Neuraly.
Whats Next For Diabetes Drugs And Parkinsons
Theres no doubt these findings add further evidence to the case that diabetes treatments could be a route to new and better treatments for Parkinsons, and theres lots to watch out for on the horizon.
The research team at UCL are already developing plans for further studies of exenatide involving more participants to investigate these promising signs in more detail.
And exenatide is not the only diabetes drug being investigated for Parkinsons.
There is already a trial underway in the United States of liraglutide an appetite control drug that also targets GLP-1 receptors. The trial started earlier this year and is expected to report results in 2019.
While another drug, originally developed for the treatment of diabetes called MSDC-0160, has also shown potential in the lab and clinical trials in people with Parkinsons will hopefully start soon.
Read Also: On And Off Phenomenon
Parkinson Progression Marker Initiative Online
open to eligible people ages 18 years and up
Parkinson Progression Marker Initiative Online is an observational study collecting participant reported information from people with and without Parkinson’s disease , for the goal of better understanding risk and predictive factors for PD. PPMI Online is part of the broader Parkinson Progression Marker Initiative aimed at identifying markers of disease progression for use in clinical trials of therapies to reduce progression of PD disability.
San Francisco, California
Did Enough Exenatide Reach The Brain
One reason that exenatide may not have achieved the same benefits as in the previous study is that there was a slight difference in the way exenatide was given.
In the first study, participants injected exenatide twice a day over a 12 month period. In this current study, the participants injected a different formulation of the drug once a week.
This formulation releases more gradually and aims to provide a more constant level of exenatide in the body, but its possible it isnt quite so effective.
When the researchers tested the levels of exenatide in the blood and the cerebrospinal fluid , their findings suggest that only a small percentage of the exenatide in the blood stream was successfully finding its way into the brain.
Finding drugs that can successfully cross the blood-brain-barrier, is one of the major challenges in treating conditions like Parkinsons and you can learn more about this in this excellent talk from Professor Matthew Wood.
Read Also: Parkinson Bicycle Cleveland Clinic
First A Little Background
Exenatide has been used since 2005 to treat type 2 diabetes. It works by targeting glucagon-like peptide-1 receptors in the pancreas, which causes insulin to be released. GLP-1 receptors are also found in the brain, and lab-based experiments have suggested that activating them can boost the function of dopamine connections, have anti-inflammatory properties, improve energy production, and switch on cell survival signals.
In 2013, an exploratory trial of exenatide for Parkinsons reported promising results.
But because there was no placebo group, both the participants and researchers knew who was receiving exenatide and who wasnt. This meant that the placebo effect could have been responsible for these positive findings and more research was required.
Importance Of The Patient Community Input
The primary task for the iLCT committee is appropriate drug selection. An important part of the deliberation is focused on safety and considerable thought is also given to patient wellbeing. At every iLCT meeting, patient advocates are invited as representatives for the PD community, and their input to the discussion is sought-after and greatly valued. For all drugs considered, special consideration must be given to whether any particular therapy is appropriate as a potential treatment for the cohort of interest taking into account the practicalities of mobility and the predominant age bracket of the patients. Factors such as drug formulation and frequency of dosing are sometimes discussed. Unique insights can be gained from the lived experience provided by the patient advocates, and this can impact which of the agents considered by the committee are eventually selected to enter clinical trials.
You May Like: Does Vitamin B12 Help Parkinson’s
Exenatide Found To Have Positive Effect On Mood
Further analysis of the results from a recent clinical trial of the type 2 diabetes drug exenatide in people with Parkinson’s has revealed encouraging signs of a positive effect on mood.
Results from this phase 2 clinical trial of exenatide were published in August 2017. The initial analysis showed positive signs that exenatide may slow the progression of Parkinson’s, but the researchers found the treatment had no significant effects on non-motor symptoms and quality of life.
However, this latest analysis, which focused only on symptoms related to mood, suggests that exenatide may improve mood, apathy and emotional-wellbeing when compared to a placebo treatment.
If Exenatide Has A Real Effect What Might Be The Mechanism
Taking into account all the preceding caveats, it is important to discuss how exenatide might be influencing the MDS UPDRS part 3 OFF scores. The impact of exenatide may be interpreted as a simple symptomatic effect. This does not necessarily mean that exenatide is directly stimulating dopamine receptors, but perhaps it might be impacting on dopaminergic signaling in other ways one proposal is that it might have a previously unrecognized effect on L-dopa pharmacokinetics.
In support of this type of symptomatic mechanism of action, the exenatide trials have indicated that clinical effects are detectable within the first 12 weeks of treatment. It is hard to imagine that this early acute effect relates to slowing down of a neurodegenerative process when the typical rate of progression in PD is approximately only 3 UPDRS points per year. Furthermore, the magnitude of the difference between exenatide and placebo groups was greater during the period of continued exposure than 12 weeks after exenatide withdrawal. Therefore exenatide seems to have at least some symptomatic effect in PD, although this might require concomitant L-Dopa therapy.
Fig.1
Also Check: Prayers For Parkinson’s Disease
The Exenatide Pd Study Trial Is Now Open For Screening And Recruitment
9 August 2021
The Exenatide PD Study trial is now open for screening and recruitment
Some of you will have heard Dr Gordon Duncan speak about plans for this important UK-wide phase 3 clinical trial at our Branch meeting in 2019. In February 2020 some ERIG members visited the Wellcome Trust Clinical Research Facility at the Western General Hospital where Exenatide trial participants will be assessed and also met some of the nursing staff involved.
Gordon is now pleased to confirm that permission has been given to start the trial in Edinburgh. The Exenatide PD Study trial is now open for screening and recruitment.
You can find out more about the trial here:
Privacy Overview
Deep Brain Stimulation For The Treatment Of Parkinson’s Disease
Sorry, in progress, not accepting new patients
The purpose of this study is to evaluate the safety and effectiveness of Boston Scientific’s Vercise Deep Brain Stimulation system in the treatment of patients with with advanced, levodopa-responsive bilateral Parkinson’s disease which is not adequately controlled with medication.
San Francisco, California
Sorry, accepting new patients by invitation only
An extension study for participants who have completed a prior VY-AADC01 clinical study
San Francisco, California
Recommended Reading: Similar To Parkinsons